Lines of investigation
Our lab is interested in understanding the cellular and molecular mechanisms governing the expansion of the cerebral cortex observed across mammalian evolution. The cerebral cortex is the largest structure in the brain and is responsible, among others, for the higher cognitive functions that distinguish humans from other mammals. The extraordinary growth in the size of the cerebral cortex observed across the mammalian evolutionary scale is thought to underlie the concomitant growth in intellectual capacity. This evolutionary expansion of the cerebral cortex is recapitulated during development in higher mammals, when the embryonic cerebral cortex undergoes massive growth in surface area, and folds itself in stereotypic patterns.
In recent years multiple genetic mutations have been identified as the leading cause for mental retardation or impairment of intellectual capacity in humans. These mutations have been consistently linked to defects of cortical development during embryogenesis, and functional studies in rodents have shown that these genes play essential roles in distinct aspects of cortical neuron migration or of cortical folding.
We are interested in the identification and analysis of the basic mechanisms involved in the normal expansion and folding of the cerebral cortex in higher mammals. To study this we combine genetic tools (in vitro and in vivo electroporation, viral vectors, transgenic and knock-out mice), experimental embryology, state-of-the-art imaging techniques and standard histological, cellular and molecular biology methods, using various species as experimental models. Currently, our efforts are focused on understanding the role of Cajal-Retzius cells and intermediate progenitors in the tangential vs. radial expansion of the cerebral cortex, and in the formation of gyri at stereotypic locations in the cerebral cortex during development.
Web CortEvo-scFerret developing cerebral cortex
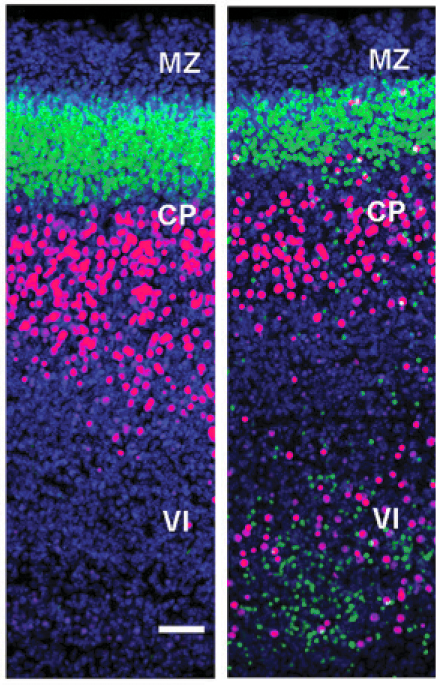
Del-Valle-Antón et al., Science Advances 2024
Representative Publications
- Multiple parallel cell lineages in the developing mammalian cerebral cortex. Del-Valle-Anton L, Amin S, Cimino D, Neuhaus F, Dvoretskova E, Fernández V, Babal YK, Garcia-Frigola C, Prieto-Colomina A, Murcia-Ramón R, Nomura Y, Cárdenas A, Feng C, Moreno-Bravo, J.A, Götz, M, Mayer C and Borrell V. Science Advances. 2024 10 (13), eadn9998 https://doi.org/10.1126/sciadv.adn9998
- Gene regulatory landscape of cerebral cortex folding Singh A, Del-Valle-Anton L, de Juan Romero C, Zhang Z, Fernández Ortuño E, Mahesh A, Espinós A, Soler R, Cárdenas A, Fernández V, Lusby R, Tiwari V K and Borrell V. Science Advances. 2024 10(23), eadn1640 https://doi.org/10.1126/sciadv.adn1640
- Epi-regulate my brain: unlocking mechanisms of brain growth evolution. Virginia Fernández and Víctor Borrell. EMBO J. 2024 43: 1385 - 1387 https://doi.org/10.1038/s44318-024-00083-8
- Keep calm and make neurons: The effects of glucocorticoids on human cortical neurogenesis. Virginia Fernández, Víctor Borrell Neuron. 2024 112(9): p1373-1375 https://doi.org/10.1016/j.neuron.2024.04.004
- Developmental mechanisms of gyrification. Fernández V, Borrell V. Curr Opin Neurobiol. 2023 80: 102711 https://10.1016/j.conb.2023.102711
- Structural basis of envelope and phase intrinsic coupling modes in the cerebral cortex. Messé A, Hollensteiner KJ, Delettre C, Dell L-A, Pieper F, Nentwig LJ, Galindo-Leon EE, Larrat B, Mériaux S, Mangin JF, Reillo I, de Juan Romero C, Borrell V, Engler G, Toro R, Engel AK, Hilgetag CC Neuroimage. 2023 276: 120212 https://doi.org/10.1016/j.neuroimage.2023.120212
- Theta/gamma Co-modulation Disruption After NMDAr Blockade by MK-801 Is Associated with Spatial Working Memory Deficits in Mice. Abad-Perez P, Molina-Payá F.J., Martínez-Otero L, Borrell V, Redondo RL, Brotons-Mas JR . Neuroscience. 2023 519: 162-176 https://doi.org/10.1016/j.neuroscience.2023.03.022
- Secondary loss of miR-3607 reduced cortical progenitor amplification during rodent evolution Chinnappa K, Cárdenas A, Prieto-Colomina A, Villalba A, Márquez-Galera Á, Soler R, Nomura Y, Llorens E, Tomasello U, López-Atalaya JP, Borrell V Sci Adv 2022 8(2):eabj4010 https://doi.org/10.1126/sciadv.abj4010
- Specific contribution of Reelin expressed by Cajal–Retzius cells or GABAergic interneurons to cortical lamination Alba Vílchez-Acosta, Yasmina Manso, Adrián Cárdenas, Alba Elias-Tersa, Magdalena Martínez-Losa, Marta Pascual, Manuel Álvarez-Dolado, Angus C. Nairn, Víctor Borrell, Eduardo Soriano PNAS 2022 119 (37): e2120079119 https://doi.org/10.1073/pnas.2120079119
- Repression of Irs2 by let-7 miRNAs is essential for homeostasis of the telencephalic neuroepithelium Fernández V, Martínez-Martínez MÁ, Prieto-Colomina A, Cárdenas A, Soler R, Dori M, Tomasello U, Nomura Y, López-Atalaya JP, Calegari F, Borrell V EMBO J 2020 39(21):e105479 https://doi.org/10.15252/embj.2020105479
- Mouse cortical organoids reveal key functions of p73 isoforms: TAp73 governs the establishment of the archetypical ventricular-like zones while DNp73 is central in the regulation of neural cell fate. Hugo Alonso-Olivares, Margarita M. Marques, Anna Prieto-Colomina, Lorena López-Ferreras, Nicole Martínez-García, Alberto Vázquez-Jiménez, Victor Borrell, Maria C. Marin , Rosalia Fernandez-Alonso Front. Cell Dev. Biol. 2024 12 , Sept. 2024 https://doi.org/10.3389/fcell.2024.1464932
- Keep calm and make neurons: The effects of glucocorticoids on human cortical neurogenesis. Virginia Fernández, Víctor Borrell Neuron. 2024 112(9): p1373-1375 https://doi.org/10.1016/j.neuron.2024.04.004
- Epi-regulate my brain: unlocking mechanisms of brain growth evolution. Virginia Fernández and Víctor Borrell. EMBO J. 2024 43: 1385 - 1387 https://doi.org/10.1038/s44318-024-00083-8
- Gene regulatory landscape of cerebral cortex folding Singh A, Del-Valle-Anton L, de Juan Romero C, Zhang Z, Fernández Ortuño E, Mahesh A, Espinós A, Soler R, Cárdenas A, Fernández V, Lusby R, Tiwari V K and Borrell V. Science Advances. 2024 10(23), eadn1640 https://doi.org/10.1126/sciadv.adn1640
- Multiple parallel cell lineages in the developing mammalian cerebral cortex. Del-Valle-Anton L, Amin S, Cimino D, Neuhaus F, Dvoretskova E, Fernández V, Babal YK, Garcia-Frigola C, Prieto-Colomina A, Murcia-Ramón R, Nomura Y, Cárdenas A, Feng C, Moreno-Bravo, J.A, Götz, M, Mayer C and Borrell V. Science Advances. 2024 10 (13), eadn9998 https://doi.org/10.1126/sciadv.adn9998
- Neocortical Neurogenesis in Amniote Evolution. Virginia Fernández, Víctor Borrell Neocortical Neurogenesis in Development and Evolution. 2023 Chapter 5: 83-105; Book Editor(s):Wieland Huttner https://doi.org/10.1002/9781119860914.ch5
- Theta/gamma Co-modulation Disruption After NMDAr Blockade by MK-801 Is Associated with Spatial Working Memory Deficits in Mice. Abad-Perez P, Molina-Payá F.J., Martínez-Otero L, Borrell V, Redondo RL, Brotons-Mas JR . Neuroscience. 2023 519: 162-176 https://doi.org/10.1016/j.neuroscience.2023.03.022
- Structural basis of envelope and phase intrinsic coupling modes in the cerebral cortex. Messé A, Hollensteiner KJ, Delettre C, Dell L-A, Pieper F, Nentwig LJ, Galindo-Leon EE, Larrat B, Mériaux S, Mangin JF, Reillo I, de Juan Romero C, Borrell V, Engler G, Toro R, Engel AK, Hilgetag CC Neuroimage. 2023 276: 120212 https://doi.org/10.1016/j.neuroimage.2023.120212
- Developmental mechanisms of gyrification. Fernández V, Borrell V. Curr Opin Neurobiol. 2023 80: 102711 https://10.1016/j.conb.2023.102711
- miR-137 and miR-122, two outer subventricular zone non-coding RNAs, regulate basal progenitor expansion and neuronal differentiation. Tomasello U, Klingler E, Niquille M, Mule N, Santinha AJ, de Vevey L, Prados J, Platt RJ, Borrell V, Jabaudon D, Dayer A Cell Rep. 2022 38(7): art 110381 https://doi.org/10.1016/j.celrep.2022.110381
- Evolution of genetic mechanisms regulating cortical neurogenesis. Espinós, A., Fernández-Ortuño, E., Negri, E., Borrell, V. Dev Neurobiol . 2022 82 (5): 428-453 Review https://doi.org/10.1002/dneu.22891
- Specific contribution of Reelin expressed by Cajal–Retzius cells or GABAergic interneurons to cortical lamination Alba Vílchez-Acosta, Yasmina Manso, Adrián Cárdenas, Alba Elias-Tersa, Magdalena Martínez-Losa, Marta Pascual, Manuel Álvarez-Dolado, Angus C. Nairn, Víctor Borrell, Eduardo Soriano PNAS 2022 119 (37): e2120079119 https://doi.org/10.1073/pnas.2120079119
- Folding brains: from development to disease modeling. Del Valle Anton L, Borrell V. Physiol Rev. 2022 102(2): 511-550 - Review https://doi.org/10.1152/physrev.00016.2021
- Secondary loss of miR-3607 reduced cortical progenitor amplification during rodent evolution Chinnappa K, Cárdenas A, Prieto-Colomina A, Villalba A, Márquez-Galera Á, Soler R, Nomura Y, Llorens E, Tomasello U, López-Atalaya JP, Borrell V Sci Adv 2022 8(2):eabj4010 https://doi.org/10.1126/sciadv.abj4010
- A protocol for in ovo electroporation of chicken and snake embryos to study forebrain development Cardenas A, Borrell V STAR Protoc 2021 2(3):100692 https://doi.org/10.1016/j.xpro.2021.100692
- Editorial: The Extracellular Environment in Controlling Neuronal Migration During Neocortical Development Hirota Y, Ohtaka-Maruyama C, Borrell V Front Cell Dev Biol 2021 9:673825 https://doi.org/10.3389/fcell.2021.673825
- MiRNAs in early brain development and pediatric cancer: At the intersection between healthy and diseased embryonic development Prieto-Colomina A, Fernández V, Chinnappa K, Borrell V BioEssays 2021 43(7):e2100073 https://doi.org/10.1002/bies.202100073
- Protomapped by the pros: Proneural factors pattern cortex folding Borrell, Victor Neuron 2021 109(18):2797 https://doi.org/10.1016/j.neuron.2021.08.007
- The regulation of cortical neurogenesis Villalba A, Gotz M Borrel, V Curr Top Dev Biol 2021 5,917361111 https://doi.org/10.1016/bs.ctdb.2020.10.003
- The regulation of cortical neurogenesis Villalba A, Goetz M, Borrell V Curr Top Dev Biol 2021 5,917361111 https://doi.org/10.1016/bs.ctdb.2020.10.003
- Molecular and cellular evolution of corticogenesis in amniotes Cárdenas A, Borrell V Cell Mol Life Sci 2020 77(8):1435 https://doi.org/10.1007/s00018-019-03315-x
- Repression of Irs2 by let-7 miRNAs is essential for homeostasis of the telencephalic neuroepithelium Fernández V, Martínez-Martínez MÁ, Prieto-Colomina A, Cárdenas A, Soler R, Dori M, Tomasello U, Nomura Y, López-Atalaya JP, Calegari F, Borrell V EMBO J 2020 39(21):e105479 https://doi.org/10.15252/embj.2020105479
- The Extracellular Matrix in the Evolution of Cortical Development and Folding Amin S, Borrell V Front Cell Dev Biol 2020 8:604448 https://doi.org/10.3389/fcell.2020.604448
- Comparison between diffusion MRI tractography and histological tract-tracing of cortico-cortical structural connectivity in the ferret brain Delettre C, Messé A, Dell LA, Foubet O, Heuer K, Larrat B, Meriaux S, Mangin JF, Reillo I, de Juan Romero C, Borrell V, Toro R, Hilgetag CC Network Neurosci 2019 3(4):1038 https://doi.org/10.1162/netn_a_00098
- A Retino-retinal Projection Guided by Unc5c Emerged in Species with Retinal Waves Murcia-Belmonte V, Coca Y, Vegar C, Negueruela S, de Juan Romero C, Valiño AJ, Sala S, DaSilva R, Kania A, Borrell V, Martinez LM, Erskine L, Herrera E Current Biology 2019 29(7):1149 https://doi.org/10.1016/j.cub.2019.02.052
- Deconstructing cortical folding: genetic, cellular and mechanical determinants Llinares-Benadero C, Borrell V Nat Rev Neurosci 2019 20(3):161 https://doi.org/10.1038/s41583-018-0112-2
- Extensive branching of radially-migrating neurons in the mammalian cerebral cortex Martínez-Martínez MÁ, Ciceri G, Espinós A, Fernández V, Marín O, Borrell V Journal of Comparative Neurology 2019 527(10):1558 https://doi.org/10.1002/cne.24597
- Recent advances in understanding neocortical development Borrell V F1000Res 2019 8:F1000 Faculty Rev-1791 https://doi.org/10.12688/f1000research.20332.1
- The centrosome protein AKNA regulates neurogenesis via microtubule organization Camargo Ortega G, Falk S, Johansson PA, Peyre E, Broix L, Sahu SK, Hirst W, Schlichthaerle T, De Juan Romero C, Draganova K, Vinopal S, Chinnappa K, Gavranovic A, Karakaya T, Steininger T, Merl-Pham J, Feederle R, Shao W, Shi SH, Hauck SM, Jungmann R, Bradke F, Borrell V, Geerlof A, Reber S, Tiwari VK, Huttner WB, Wilsch-Bräuninger M, Nguyen L, Götz M Nature 2019 567(7746):113 https://doi.org/10.1038/s41586-019-0962-4
- Evolution of Cortical Neurogenesis in Amniotes Controlled by Robo Signaling Levels Cardenas A, Villalba A, de Juan Romero C, Pico E, Kyrousi C, Tzika AC, Tessier-Lavigne M, Ma L, Drukker M, Cappello S, Borrell V Cell 2018 174(3):590 https://doi.org/10.1016/j.cell.2018.06.007
- How Cells Fold the Cerebral Cortex Borrell V J Neurosci 2018 38(4):776 https://doi.org/10.1523/JNEUROSCI.1106-17.2017
- Skill Learning Modulates RNA Pol II Poising at Immediate Early Genes in the Adult Striatum Galvão-Ferreira P, Lipinski M, Santos F, Barco A, Costa RM eNeuro 2017 4(2):UNSP e0074-17.2017 https://doi.org/10.1523/ENEURO.0074-17.2017
- A Complex Code of Extrinsic Influences on Cortical Progenitor Cells of Higher Mammals Reillo I, de Juan Romero C, Cardenas A, Clasca F, Martinez-Martinez MA, Borrell V Cereb Cortex 2017 27(9):4586 https://doi.org/10.1093/cercor/bhx171
- Deletion of Dlk2 increases the vulnerability to anxiety-like behaviors and impairs the anxiolytic action of alprazolam Navarrete F, Garcia-Gutierrez MS, Laborda J, Manzanares J Psychoneuroendocrinology 2017 85:134 https://doi.org/10.1016/j.psyneuen.2017.08.015
- Lack of IL-1R8 in neurons causes hyperactivation of IL-1 receptor pathway and induces MECP2-dependent synaptic defects Tomasoni R, Morini R, Lopez-Atalaya JP, Corradini I, Canzi A, Rasile M, Mantovani C, Pozzi D, Garlanda C, Mantovani A, Menna E, Barco A, Matteoli M Elife 2017 6:e21735 https://doi.org/10.7554/eLife.21735
- Regulation of Cerebral Cortex Folding by Controlling Neuronal Migration via FLRT Adhesion Molecules del Toro D, Ruff T, Cederfjaell E, Villalba A, Seyit-Bremer G, Borrell V, Klein R Cell 2017 169(4):621 https://doi.org/10.1016/j.cell.2017.04.012
- A restricted period for formation of outer subventricular zone defined by Cdh1 and Trnp1 levels Martinez-Martinez Ma, De Juan Romero C, Fernandez V, Cardenas A, Götz M, Borrell V Nat Commun 2016 7:11812 https://doi.org/10.1038/ncomms11812
- Cerebral cortex expansion and folding: what have we learned? Fernandez V, Llinares-Benadero C, Borrell V EMBO J 2016 35(10):1021 https://doi.org/10.15252/embj.201593701
- Coevolution of radial glial cells and the cerebral cortex De Juan Romero C, Borrell V Glia 2015 63(8):1303 https://doi.org/10.1002/glia.22827
- Discrete domains of gene expression in germinal layers distinguish the development of gyrencephaly De Juan Romero C, Bruder C, Tomasello U, Sanz-Anquela JM, Borrell V EMBO J 2015 34(14):1859 https://doi.org/10.15252/embj.201591176
- Transcriptional Profiling of Hypoxic Neural Stem Cells Identifies Calcineurin-NFATc4 Signaling as a Major Regulator of Neural Stem Cell Biology Moreno M, Fernandez V, Monllau JM, Borrell V, Lerin C, de la Iglesia N Stem Cell Rep 2015 5(2):157 https://doi.org/10.1016/j.stemcr.2015.06.008
- Mechanisms of brain evolution: Regulation of neural progenitor cell diversity and cell cycle length Borrell V, Calegari F Neurosci Res 2014 86:14 https://doi.org/10.1016/j.neures.2014.04.004
- Mutations in Eml1 lead to ectopic progenitors and neuronal heterotopia in mouse and human Kielar M, Tuy FPD, Bizzotto S, Lebrand C, De Juan Romero C, Poirier K, Oegema R, Mancini GM, Bahi-Buisson N, Olaso R, LeMoing A, Boutourlinsky K, Boucher D, Carpentier W, Berquin P, Deleuze J, Belvindrah J, Borrell V, Welker E, Chelly J, Croquelois A, Francis F Nat Neurosci 2014 17(7):923 https://doi.org/10.1038/nn.3729
- Phenotyping the central nervous system of the embryonic mouse by magnetic resonance microscopy Martinez-Martinez MA, Pacheco-Torres J, Borrell V, Canals S NeuroImage 2014 97:95 https://doi.org/10.1016/j.neuroimage.2014.04.043
- Role of Radial Glia cells in cerebral cortex folding Borrell V, Goetz M Curr Opin Neurobiol 2014 27:39 https://doi.org/10.1016/j.conb.2014.02.007
- Regulation of cerebral cortex size and folding by expansion of basal progenitors Nonaka-Kinoshita M, Reillo I, Artegiani B, Martinez-Martinez MA, Nelson M, Borrell V, Calegari F EMBO J 2013 32(13):1817 https://doi.org/10.1038/emboj.2013.96
- Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type Pilz GA, Shitamukai A, Reillo I, Pacary E, Schwausch J, Stahl R, Ninkovic J, Snipper HJ, Clevers H, Godinho L,Guillemot F, Borrell V, Matsuzaki F, Goetz M Nat Commun 2013 4:2125 https://doi.org/10.1038/ncomms3125
- Contact repulsion controls the dispersion and final distribution of Cajal-Retzius cells Villar-Cerviño V, Molano-Mazon M, Catchpole T, Valdeolmillos M, Henkemeyer M, Martinez LM, Borrell V, Marin O Neuron 2013 77(3):457 https://doi.org/10.1016/j.neuron.2012.11.023
- Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate, Stahl R, Walcher T, De Juan C, Pilz GA, Capello S, Irmler M, Sanz-Anquela JM, Beckers J, Blum R, Borrell V, Gotz M Cell 2013 153(3):535 https://doi.org/10.1016/j.cell.2013.03.027
- Tau Isoform with Three Microtubule Binding Domains is a Marker of New Axons Generated from the Subgranular Zone in the Hippocampal Dentate Gyrus: Implications for Alzheimer’s Disease Llorens-Martin M, Teixeira CM, Fuster-Matanzo A, Jurado-Arjona J, Borrell V, Soriano E, Avila J, Hernandez F J Alzheimers Dis 2012 29(4):921 https://doi.org/10.3233/JAD-2012-112057
- Slit/Robo Signaling Modulates the Proliferation of Central Nervous System Progenitors Borrell V, Cardenas A, Ciceri G, Galceran J, Flames N, Pla R, Nobrega-Pereira S, Garcia-Frigola C, Peregrin S, Zhao Z, Ma L, Tessier-Lavigne M, Marin O Neuron 2012 76(2):338 https://doi.org/10.1016/j.neuron.2012.08.003
- Germinal Zones in the Developing Cerebral Cortex of Ferret: Ontogeny, Cell Cycle Kinetics, and Diversity of Progenitors Reillo I, Borrell V Cereb Cortex 2012 22(9):2039 https://doi.org/10.1093/cercor/bhr284
- Emerging roles of neural stem cells in cerebral cortex development and evolution Borrell V, Reillo I Dev Neurobiol 2012 72(7):955 https://doi.org/10.1002/dneu.22013
- Cell-Autonomous Inactivation of the Reelin Pathway Impairs Adult Neurogenesis in the Hippocampus Teixeira CM, Kron MM, Masachs N, Zhang HL, Lagace DC, Martinez A, Reillo I, Duan X, Bosch C, Pujadas L, Brunso L, Song HJ, Eisch AJ, Borrell V, Howell BW, Parent JM, Soriano E J Neurosci 2012 32(35):12051 https://doi.org/10.1523/JNEUROSCI.1857-12.2012
- Abundant Occurrence of Basal Radial Glia in the Subventricular Zone of Embryonic Neocortex of a Lissencephalic Primate, the Common Marmoset Callithrix jacchus Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, Okano H, Huttner WB, Borrell V Cereb Cortex 2012 22(2):469 https://doi.org/10.1093/cercor/bhr301
- Maldevelopment of the cerebral cortex in the surgically induced model of myelomeningocele: implications for fetal neurosurgery Encinas JL, Garcia-Cabezas MA, Barkovich J, Fontecha CG, Peiro JL, Soto GM, Borrell V, Reillo I, Lopez-Santamaria M, Tovar JA, Farmer DL J Pediatr Surg 2011 46(4):713 https://doi.org/10.1016/j.jpedsurg.2010.11.028
- Developmental Sculpting of Dendritic Morphology of Layer 4 Neurons in Visual Cortex: Influence of Retinal Input Callaway EM, Borrell V J Neurosci 2011 31(20):7456 https://doi.org/10.1523/JNEUROSCI.5222-10.2011
- A Role for Intermediate Radial Glia in the Tangential Expansion of the Mammalian Cerebral Cortex Reillo I, De Juan Romero C, Garcia-Cabezas MA, Borrell V Cereb Cortex 2011 21(7):1674 https://doi.org/10.1093/cercor/bhq238
- In vivo gene delivery to the postnatal ferret cerebral cortex by DNA electroporation Borrell V J Neurosci Methods 2010 186(2):186 https://doi.org/10.1016/j.jneumeth.2009.11.016

 Español
Español