Our Neurotropic Vectors Unit is devoted to the production of delivery vectors of viral origin for the study of the nervous system in health and disease.
In recent years the delivery of molecular tools into neurons has become an essential approach to understand the mechanisms underlying brain function and brain disorders.
Genetically engineered viruses have become ideal vectors for introducing these tools into brain cells allowing neuroscientists unprecedented control over cells and circuits.
To facilitate the use of these state-of-the-art methodologies by our neuroscientists, the Vector Unit centralizes the process of producing and distributing neurotropic vectors.
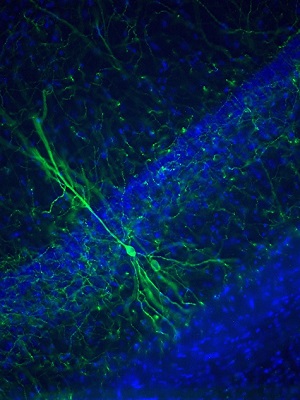
Hippocampal cell infected with a EGFP expressing AAV.(fig.1)
We currently offer custom-made Adeno-associated virus (AVV), Retrovirus (RV) and Lentivirus (LV)-based vector preparations for neuroscience purposes.
AAV specifications:
- Plasmids: The Unit provides plasmids encoding for the most common capsids (1,2,5,8,9,11, PHP.eB, PHP.S, 2 retro, 9p31, ROOT) and helper genes. Because it is important to pick the viral vector that best suits the specific needs, we encourage the users to contact the Unit for advice. Specific gene/construct of interest plasmids should be provided by the user.
- Production: Our AAV vector production method utilizes the triple transfection protocol in HEK293 cells followed by purification using iodixanol gradients, to achieve greater enrichment in infecting viral particles, and a final concentration step. Our titering method is based on Q-PCR.
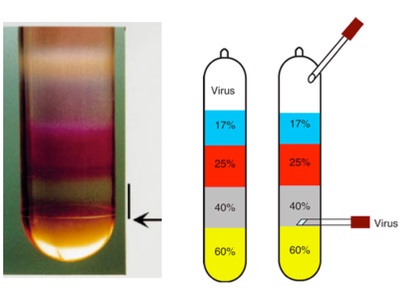
Iodixanol purification (Grieger et al, 2006)
- Safety: AAV vectors are biosafety level 1 and consist of recombinant transgene sequences (e.g., reporter gene) flanked by the AAV inverted terminal repeats. The removal of the majority of viral structural genes renders the vector replication-defective and dependent on an AAV helper virus.
- Handling and storage: All viral vectors are delivered frozen and should be stored at -80° C for long term storage. Vectors can be stored for short periods of time at -20 or +4°C. Whenever possible, vectors should be aliquoted into single use portions to avoid repeated freeze/thaw cycles. Please aliquot in at least 5ul per tube and use low protein binding tubes to avoid loss of virus.
Vectors are ready to use upon reception. If you need to dilute the virus, you may use PBS, but do so ONLY prior to use. Do not dilute unless ready to use.
RV and LV specifications:
- Plasmids: The Unit provides plasmids encoding for the most common envelope glycoproteins (VSVG and Rabies Glycoprotein) and helper genes (gag, pol). Because it is important to pick the viral vector that best suits the specific needs, we encourage the users to contact the Unit for advice. Specific gene/construct of interest plasmids should be provided by the user.
- Production: Our RV and LV vectors production method utilizes the triple transfection protocol in HEK293-T cells followed by filtration and ultracentrifugation to achieve high concentrations compatible with their use in vivo. Our titering method is based on Q-RT-PCR.
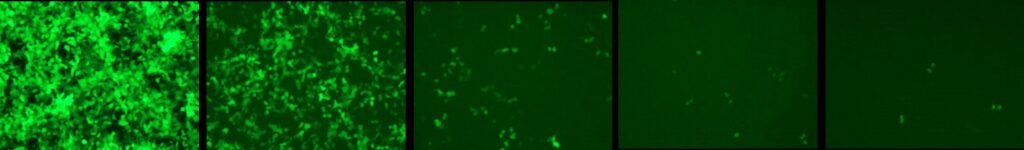
HEK293 cells infected with serial dilutions of a EGFP expressing LV
- Safety: RV and LV vectors are biosafety level 2 and consist of recombinant transgene sequences (e.g., reporter gene) flanked by the RV or LV long terminal repeats (LTR). The removal of the majority of viral structural genes renders the vector replication-defective. RV and LV vector sequences integrate randomly on the host genome.
- Handling and storage: All viral vectors are delivered frozen and should be stored at -80° C for long term storage. Vectors can be stored for short periods of time at -20. Whenever possible, vectors should be aliquoted into single use portions to avoid repeated freeze/thaw cycles. Please aliquot in at least 5ul per tube and use low protein binding tubes to avoid loss of virus.
Vectors are ready to use upon reception. If you need to dilute the virus, you may use PBS, but do so ONLY prior to use. Do not dilute unless ready to use.
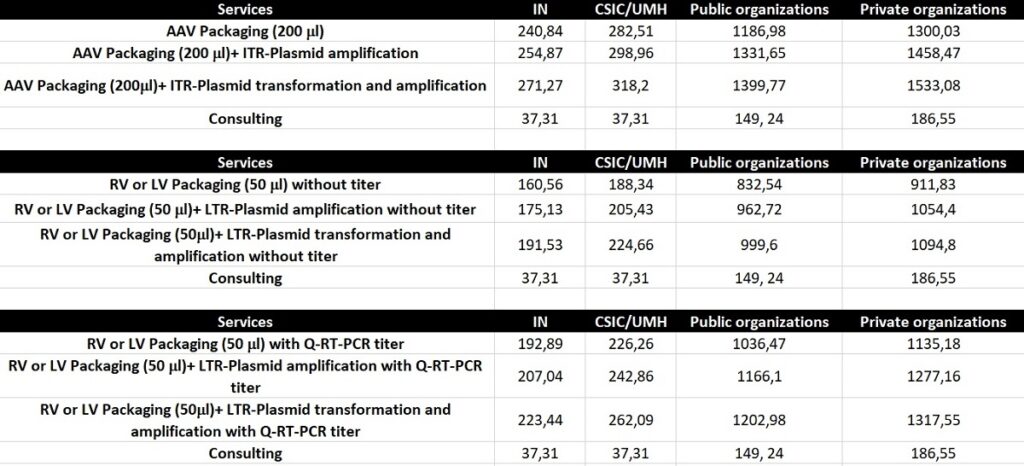
The cost of each prep will depend on the specific needs of the user. Basic rates are listed below. Please contact the Unit for a quote.
email: vectores@umh.es
Table 1. Price list (€) 2025
Out Viral Vector Unit space includes separate suites for DNA and virus work allocated respectively, at:
- Lab 203.
- Biosafety (NCB2) lab.
Technical:

María de los Ángeles Hernández Vellisca
maria.hernandezv@umh.es »
ext. 9289
For general communications, inquires about our services, ordering, training, protocols, etc… please, contact us at: vectores@umh.es

The Unit has all the basic equipment necessary for viral vector production (including a BSL-2 facility equipped with tissue culture hoods and incubators, benchtop centrifuges, ultracentrifuges) and titration (molecular biology laboratory, Quant-Studio machine PCR).


Photos: Instituto de Neurociencias

 Español
Español
