Lines of investigation
Sensory terminals of the skin and mucosae are subjected to a continuous bombardment of physical and chemical stimuli. These stimuli must be transformed into a code of electrical signals that is relayed to the central nervous system to evoke conscious sensations. Many details of this biological process, known as sensory transduction, are still elusive. This is specially true for stimuli that cause tissue injury and underlie the sensation of pain. Pain is a very frequent medical condition, with enormous costs and severe social impact in our communities.
Our research group is interested in the cellular and molecular mechanisms underlying the transduction on low and high threshold mechanical, thermal (cold and warm) and chemical stimuli (both endogeneous and exogeneous mediators) by primary sensory neurons. We also seek to determine modulatory mechanisms in the responses and search for new potential therapeutic targets for the control of pain.
Current studies include the transcriptome profiling of subpopulations of primary sensory neurons in different models of chronic pain, structure and function of TRP and Piezo2 channels and the optogenetic interrogation of thermosensory and mechanosensory circuits. Additional efforts are devoted also to characterizing novel TRP channel modulators and their impact on different disease models.
In these studies, we use different techniques: molecular biology and genetic manipulation, RNASeq, pharmacology, immunocytochemistry, in vitro and in vivo electrophysiology, piezoelectric activation of mechanosensitive channels, imaging techniques like intracellular calcium measurements and TIRF, FRET, FRAP and behavioural tests in rodents (nociception tests). Recent addition to our technical palette includes the selective expression of light-sensitive ion channels with sensory-specific Cre-driver lines (TRPM8, Advilin, Nav1.8, TRPA1, Piezo2, etc) to manipulate sensory activity in vivo and in vitro (optogenetics).
Representative Publications
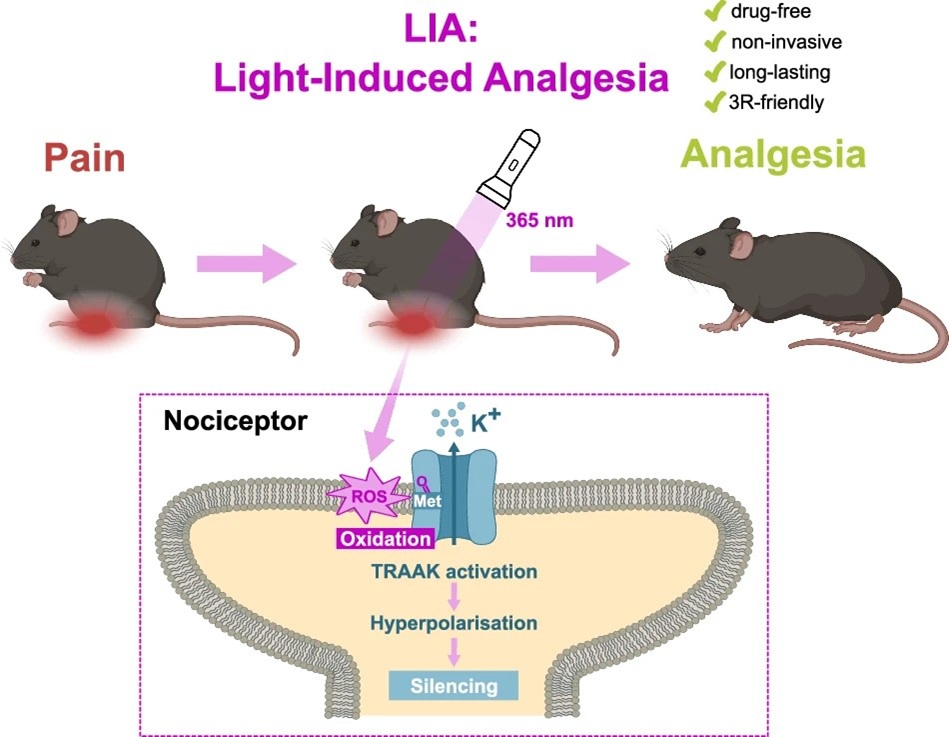
- Light-induced analgesia provides a drug-free optical method for pain relief via activation of TRAAK k+ channels. Marion Bied, Arnaud Landra-Willm, Anne Amandine Chassot, Edward Francisco Mendez-Otalvaro, Benjamin Sueur, Kilian Roßmann, Elvira de la Peña, Pascal Fossat, Stephen J. Tucker, Jacques Noël, Wojciech Kopec, Felix Viana, Johannes Broichhagen, Eric Boué-Grabot & Guillaume Sandoz. Nat Commun. 2026 17, 620 https://doi.org/10.1038/s41467-025-67819-w
- Transduction Mechanisms for Cold Temperature in Mouse Trigeminal and Vagal Ganglion Neurons Innervating Different Peripheral Organs. Katharina Gers-Barlag, Ana Gómez Del Campo , Pablo Hernández-Ortego ,Eva Quintero,Félix Viana. Acta Physio. 2025 241(11): e70111 https://doi.org/10.1111/apha.70111
- The ion channel TRPM8 is a direct target of the immunosuppressant rapamycin in primary sensory neurons. Arcas JM, Oudaha K, González A, Fernández-Trillo J, Peralta FC, Castro-Marsal J, Poyraz S, Tabener F, Sala S, de la Peña E, gomis A, Viana F. British Journal of Pharmacology. 2024 First published: 13 May 2024 https://doi.org/10.1111/bph.16402
- Proper Frequency of Perinatal Retinal Waves Is Essential for the Precise Wiring of Visual Axons in Nonimage-Forming Nuclei. Negueruela S, Morenilla-Palao C, Herrera M, Coca Y, Florez-Paz D, López-Cascales MT, Gomis A, Herrera E*. J Neurosci. 2024 44 (40): e1408232024 https://doi.org/10.1523/JNEUROSCI.1408-23.2024
- TRPA1 modulation by Sigma-1 receptor prevents oxaliplatin-induced painful peripheral neuropathy Marcotti, A., Fernández-Trillo, J., González, A., Vizcaíno-Escoto, M., Ros-Arlanzón, P., Romero, L., Vela, J. M., Gomis, A., Viana, F., & de la Peña, E Brain 2023 146 (2): 475-491 https://doi.org/10.1093/brain/awac273
- Detecting Warm Temperatures Is a Cool Kind of Thing Gomez Del Campo A, Viana F Neuron 2020 106(5):712 https://doi.org/10.1016/j.neuron.2020.05.009
- Piezo2 mediates low-threshold mechanically-evoked pain in the cornea Fernández-Trillo J, Florez-Paz D, Iñigo-Portugués A, González-González O, Del Campo AG, González A, Viana F, Belmonte C, Gomis A J Neurosci 2020 40(47):8976 https://doi.org/10.1523/JNEUROSCI.0247-20.2020
- The immunosuppressant macrolide tacrolimus activates cold-sensing TRPM8 channels Miguel Arcas J, Gonzalez A, Gers-Barlag K, Gonzalez-Gonzalez O, Bech F, Demirkhanyan L, Zakharian E, Belmonte C, Gomis A, Viana F Journal of Neuroscience 2019 39(6):949 https://doi.org/10.1523/JNEUROSCI.1726-18.2018
- Joint nociceptor nerve activity and pain in an animal model of acute gout and its modulation by intra-articular hyaluronan Marcotti A, Miralles A, Dominguez E, Pascual E, Gomis A, Belmonte C, de la Pena E Pain 2018 159(4):739 https://doi.org/10.1097/j.pain.0000000000001137
- A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons Florez-Paz D, Bali KK, Kuner R, Gomis A Sci Rep 2016 6:25923 https://doi.org/10.1038/srep25923
- Light-induced analgesia provides a drug-free optical method for pain relief via activation of TRAAK k+ channels. Marion Bied, Arnaud Landra-Willm, Anne Amandine Chassot, Edward Francisco Mendez-Otalvaro, Benjamin Sueur, Kilian Roßmann, Elvira de la Peña, Pascal Fossat, Stephen J. Tucker, Jacques Noël, Wojciech Kopec, Felix Viana, Johannes Broichhagen, Eric Boué-Grabot & Guillaume Sandoz. Nat Commun. 2026 17, 620 https://doi.org/10.1038/s41467-025-67819-w
- Transduction Mechanisms for Cold Temperature in Mouse Trigeminal and Vagal Ganglion Neurons Innervating Different Peripheral Organs. Katharina Gers-Barlag, Ana Gómez Del Campo , Pablo Hernández-Ortego ,Eva Quintero,Félix Viana. Acta Physio. 2025 241(11): e70111 https://doi.org/10.1111/apha.70111
- Proper Frequency of Perinatal Retinal Waves Is Essential for the Precise Wiring of Visual Axons in Nonimage-Forming Nuclei. Negueruela S, Morenilla-Palao C, Herrera M, Coca Y, Florez-Paz D, López-Cascales MT, Gomis A, Herrera E*. J Neurosci. 2024 44 (40): e1408232024 https://doi.org/10.1523/JNEUROSCI.1408-23.2024
- The ion channel TRPM8 is a direct target of the immunosuppressant rapamycin in primary sensory neurons. Arcas JM, Oudaha K, González A, Fernández-Trillo J, Peralta FC, Castro-Marsal J, Poyraz S, Tabener F, Sala S, de la Peña E, gomis A, Viana F. British Journal of Pharmacology. 2024 First published: 13 May 2024 https://doi.org/10.1111/bph.16402
- Sex differences in thermoregulation in mammals: Implications for energy homeostasis. Carlos Fernández-Peña , Alfonso Reimúndez, Félix Viana, Victor M. Arce, Rosa Señarís. Front. Endocrinol. 2023 Sec. Neuroendocrine Science - Volume 14 (Review) https://doi.org/10.3389/fendo.2023.1093376
- The cold-sensing ion channel TRPM8 regulates central and peripheral clockwork and the circadian oscillations of body temperature. Reimúndez, A., Fernández-Peña, C., Ordás, P., Hernández-Ortego, P., Gallego, R., Morenilla-Palao, C., Navarro, J., Martín-Cora, F., Pardo-Vázquez, J.L., Schwarz, L.A., Arce, V., Viana, F., Señarís, R. Acta Physiol (Oxf) . 2023 237(3): art. e13896 https://doi.org/10.1111/apha.13896
- TRPA1 modulation by Sigma-1 receptor prevents oxaliplatin-induced painful peripheral neuropathy Marcotti, A., Fernández-Trillo, J., González, A., Vizcaíno-Escoto, M., Ros-Arlanzón, P., Romero, L., Vela, J. M., Gomis, A., Viana, F., & de la Peña, E Brain 2023 146 (2): 475-491 https://doi.org/10.1093/brain/awac273
- Reflections on the Ocular Surface: Summary of the Presentations at the 4th Coronis Foundation Ophthalmic Symposium Debate: “A Multifactorial Approach to Ocular Surface Disorders” (August 31 2021). Bron, A.J., Dogru, M., Horwath-Winter, J., Kojima, T., Kovács, I., Müller-Lierheim, W.G.K., van Setten, G.-B., Belmonte, C. Front Biosci (Landmark Ed). 2022 27(5): 142 https://doi.org/10.31083/j.fbl2705142
- Origins of direction selectivity in the primate retina. Kim, Y.J., Peterson, B.B., Crook, J.D., Joo, H.R., Wu, J., Puller, C., Robinson, F.R., Gamlin, P.D., Yau, K.-W., Viana, F., Troy, J.B., Smith, R.G., Packer, O.S., Detwiler, P.B., Dacey, D.M. Nat Commun. 2022 13(1): art 2862 https://doi.org/10.1038/s41467-022-30405-5
- Validation of Six Commercial Antibodies for the Detection of Heterologous and Endogenous TRPM8 Ion Channel Expression. Hernández-Ortego, P., Torres-Montero, R., de la Peña, E., Viana, F., Fernández-Trillo, J. Int J Mol Sci. 2022 23(24): 16164 https://doi.org/10.3390/ijms232416164
- Expression of the cold thermoreceptor TRPM8 in rodent brain thermoregulatory circuits Ordás P, Hernández-Ortego P, Vara H, Fernández-Peña C, Reimúndez A, Morenilla-Palao C, Guadaño-Ferraz A, Gomis A, Hoon M, Viana F, Señarís R J Comp Neurol 2021 529(1):234 https://doi.org/10.1002/cne.24694
- Constitutive phosphorylation as a key regulator of TRPM8 channel function Rivera B, Moreno C, Lavanderos B, Hwang JY, Fernández-Trillo J, Park KS, Orio P, Viana F, Madrid R, Pertusa M J Neurosci 2021 41(41):8475 https://doi.org/10.1523/JNEUROSCI.0345-21.2021
- Detecting Warm Temperatures Is a Cool Kind of Thing Gomez Del Campo A, Viana F Neuron 2020 106(5):712 https://doi.org/10.1016/j.neuron.2020.05.009
- Piezo2 mediates low-threshold mechanically-evoked pain in the cornea Fernández-Trillo J, Florez-Paz D, Iñigo-Portugués A, González-González O, Del Campo AG, González A, Viana F, Belmonte C, Gomis A J Neurosci 2020 40(47):8976 https://doi.org/10.1523/JNEUROSCI.0247-20.2020
- The immunosuppressant macrolide tacrolimus activates cold-sensing TRPM8 channels Miguel Arcas J, Gonzalez A, Gers-Barlag K, Gonzalez-Gonzalez O, Bech F, Demirkhanyan L, Zakharian E, Belmonte C, Gomis A, Viana F Journal of Neuroscience 2019 39(6):949 https://doi.org/10.1523/JNEUROSCI.1726-18.2018
- Deletion of the Cold Thermoreceptor TRPM8 Increases Heat Loss and Food Intake Leading to Reduced Body Temperature and Obesity in Mice Reimundez A, Fernandez-Peña C, Garcia G, Fernandez R, Ordas P, Gallego R, Pardo-Vazquez JL, Arce V, Viana F, Señaris R J Neurosci 2018 38(15):3643 https://doi.org/10.1523/JNEUROSCI.3002-17.2018
- Joint nociceptor nerve activity and pain in an animal model of acute gout and its modulation by intra-articular hyaluronan Marcotti A, Miralles A, Dominguez E, Pascual E, Gomis A, Belmonte C, de la Pena E Pain 2018 159(4):739 https://doi.org/10.1097/j.pain.0000000000001137
- Mammalian cold TRP channels: impact on thermoregulation and energy homeostasis Señarís R, Ordás P, Reimúndez A, Viana F Pflugers Arch 2018 470(5):761 https://doi.org/10.1007/s00424-018-2145-9
- Morphological and functional changes in TRPM8-expressing corneal cold thermoreceptor neurons during aging and their impact on tearing in mice Alcalde I, Íñigo-Portugués A, González-González O, Almaraz L, Artime E, Morenilla-Palao C, Gallar J, Viana F, Merayo-Lloves J, Belmonte C J Comp Neurol 2018 526(11):1859 https://doi.org/10.1002/cne.24454
- Nociceptors: thermal allodynia and thermal pain Viana, Felix THERMOREGULATION: FROM BASIC NEUROSCIENCE TO CLINICAL NEUROLOGY, PT I 2018 156:103 https://doi.org/10.1016/B978-0-444-63912-7.00006-0
- TFOS DEWS II Report Executive Summary Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, de Paiva CS, Gomes JAP, Hammitt KM, Jones L, Nichols JJ, Nichols KK, Novack GD, Stapleton FJ, Willcox MDP, Wolffsohn JS, Sullivan DA Ocul Surf 2017 15(4):802 https://doi.org/10.1016/j.jtos.2017.08.003
- TFOS DEWS II pain and sensation report Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, Dartt DA, Galor A, Hamrah P, Ivanusic JJ, Jacobs DS, McNamara NA, Rosenblatt MI, Stapleton F, Wolffsohn JS Ocul Surf 2017 15(3):404 https://doi.org/10.1016/j.jtos.2017.05.002
- A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons Florez-Paz D, Bali KK, Kuner R, Gomis A Sci Rep 2016 6:25923 https://doi.org/10.1038/srep25923
- New Insight in Cold Pain: Role of Ion Channels, Modulation, and Clinical Perspectives Lolignier S, Gkika D, Andersson D, Leipold E, Vetter I, Viana F, Noël J, Busserolles J J Neurosci 2016 36(45):11435 https://doi.org/10.1523/JNEUROSCI.2327-16.2016
- TRPA1 channels: molecular sentinels of cellular stress and tissue damage Viana, F J Physiol (London) 2016 594(15):4151 https://doi.org/10.1113/JP270935
- TRPV1 channel modulation by hyaluronan reduces pain de la Peña E, Gomis A, Ferrer-Montiel A, Belmonte C Channels (Austin) 2016 10(2):81 https://doi.org/10.1080/19336950.2015.1109300
- Hyaluronan modulates TRPV1 channel opening, reducing peripheral nociceptor activity and pain Caires R, Luis E, Taberner FJ, Fernandez-Ballester G, Ferrer-Montiel A, Balazs EA, Gomis A, Belmonte C, De la Peña E Nat Commun 2015 6:8095 https://doi.org/10.1038/ncomms9095
- TRPM8 is a neuronal osmosensor that regulates eye blinking in mice Quallo T, Vastani N, Horridge E, Gentry C, Parra A, Moss S, Viana F, Belmonte C, Andersson DA, Bevan S Nat Commun 2015 6:7150 https://doi.org/10.1038/ncomms8150
- Bidirectional modulation of thermal and chemical sensitivity of TRPM8 channels by the initial region of the N-terminal domain Pertusa M, Gonzalez A, Hardy P, Madrid R, Viana F J Biol Chem 2014 289(32):21828 https://doi.org/10.1074/jbc.M114.565994
- G protein-coupled receptor signalling potentiates the osmo-mechanical activation of TRPC5 channels Jemal I, Soriano S, Conte AL, Morenilla C, Gomis A Pflugers Arch 2014 466(8):1635 https://doi.org/10.1007/s00424-013-1392-z
- Ion Channel Profile of TRPM8 Cold Receptors Reveals a Role of TASK-3 Potassium Channels in Thermosensation Morenilla-Palao C, Luis E, Fernandez-Peña C, Quintero E, Weaver JL, Bayliss DA, Viana F Cell Rep 2014 8(5):1571 https://doi.org/10.1016/j.celrep.2014.08.003
- Plasma membranes as heat stress sensors: From lipid-controlled molecular switches to therapeutic applications Török Z, Crul T, Maresca B, Schütz GJ, Viana F, Dindia L, Piotto S, Brameshuber M, Balogh G, Peter M, Porta A, Trapani A, Gombos I, Glatz A, Gungor B, Peksel B, Vigh L Jr, Csoboz B, Horvath I, Vijayan MM, Hooper PL, Harwood JL, Vigh L Biochim Biophys Acta-Biomembranes 2014 1838(6):1594 https://doi.org/10.1016/j.bbamem.2013.12.015
- TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins Meseguer V, Alpizar YA,Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernandez-Peña C,Talavera A, Kichko T, Navia B, Sanchez A, Señaris R, Reeh P,Perez-Garcia MT, Lopez-Lopez JR, Voets T, Belmonte C, Talavera K, Viana F Nat Commun 2014 5:3125 https://doi.org/10.1038/ncomms4125
- Monoacylglycerols Activate TRPV1-A Link between Phospholipase C and TRPV1 Zygmunt PM, Ermund A, Movahed P, Andersson DA, Simonsen C, Jönsson BA, Blomgren A, Birnir B, Bevan S, Eschalier A, Mallet C, Gomis A, Högestätt ED PLoS One 2013 8(12):e81618 https://doi.org/10.1371/journal.pone.0081618
- The TFOS International Workshop on Contact Lens Discomfort: Executive Summary Nichols JJ, Willcox MD, Bron AJ, Belmonte C, Ciolino JB, Craig JP, Dogru M, Foulks GN, Jones L, Nelson JD, Nichols KK, Purslow C, Schaumberg DA, Stapleton F, Sullivan DA; members of the TFOS International Workshop on Contact Lens Discomfort Invest Ophthalmol Vis Sci 2013 54(11):7 https://doi.org/10.1167/iovs.13-13212
- Blockade of nociceptive sensory afferent activity of the rat knee joint by the bradykinin B2 receptor antagonist fasitibant Gomis A, Meini S, Miralles A, Valenti C, Giuliani S, Belmonte C, Maggi CA Osteoarthr Cartilage 2013 21(9):1346 https://doi.org/10.1016/j.joca.2013.03.013
- TRPM8 Ion Channels Differentially Modulate Proliferation and Cell Cycle Distribution of Normal and Cancer Prostate Cells Valero MLI, Mello de Queiroz F, Stuhmer W, Viana F, Pardo LA PLoS ONE 2012 7(12):e51825 https://doi.org/10.1371/journal.pone.0051825
- The Influence of Cold Temperature on Cellular Excitability of Hippocampal Networks de la Peña E, Mälkiä A, Vara H, Caires R, Ballesta JJ, Belmonte C, Viana F PLoS ONE 2012 7(12):e52475 https://doi.org/10.1371/journal.pone.0052475
- Role of Ih in the firing pattern of mammalian cold thermoreceptor endings Orio P, Parra A, Madrid R, Gonzalez O, Belmonte C, Viana F J Neurophysiol 2012 108(11):3009 https://doi.org/10.1152/jn.01033.2011
- N-Glycosylation of TRPM8 Ion Channels Modulates Temperature Sensitivity of Cold Thermoreceptor Neurons Pertusa M, Madrid R, Morenilla-Palao C, Belmonte C, Viana F J Biol Chem 2012 287(22):18218 https://doi.org/10.1074/jbc.M111.312645
- Direct inhibition of the cold-activated TRPM8 ion channel by G-alpha(q) Zhang X, Mak S, Li L, Parra A, Denlinger B, Belmonte C, McNaughton PA Nat Cell Biol 2012 14(8):850 https://doi.org/10.1038/ncb2529
- The Emerging Pharmacology of TRPM8 Channels: Hidden Therapeutic Potential Underneath a Cold Surface Mälkiä A, Morenilla-Palao C, Viana F Curr Pharm Biotechnol 2011 12(1):54 https://doi.org/10.2174/138920111793937916
- Pharmacological and functional properties of TRPM8 channels in prostate tumor cells Valero M, Morenilla-Palao C, Belmonte C, Viana F Pflugers Arch 2011 461(1):99 https://doi.org/10.1007/s00424-010-0895-0
- Membrane-tethered peptides patterned after the TRP domain (TRPducins) selectively inhibit TRPV1 channel activity Valente P, Fernandez-Carvajal A, Camprubi-Robles M, Gomis A, Quirce S, Viana F, Fernandez-Ballester G, Gonzalez-Ros JM, Belmonte C, Planells-Cases R, Ferrer-Montiel A FASEB J 2011 25(5):1628 https://doi.org/10.1096/fj.10-174433
- Chemosensory Properties of the Trigeminal System Viana F ACS Chem Neurosci 2011 2(1):38 https://doi.org/10.1021/cn100102c
- Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea Parra A, Madrid R, Echevarria D, Del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C Nat Med 2010 16(12):1396 https://doi.org/10.1038/nm.2264

 Español
Español