Lines of investigation
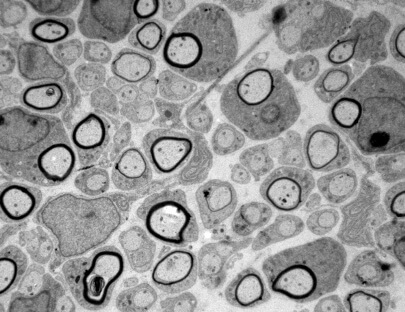
Nerve conduction velocity is inversely proportional to the electrical resistance of the axon and the capacitance of the plasma membrane that surrounds it. To increase nerve impulse velocity some invertebrates (such as squid) decreases resistance of the axon by greatly increasing its diameter. In more complex nervous systems, like higher vertebrates, this would increase by more than a hundred times the volume of the nervous system. To increase nerve conduction velocity without changing the axonal diameter (and nervous system volume) it is necessary to reduce the capacitance by increasing the thickness of the lipid membrane surrounding the axon. This has been achieved in vertebrates by depositing large amounts of plasma membrane of specialized hypertrophied neighboring cells (oligodendrocytes or Schwann cells). Rudolf Virchow first described this membrane, known as “myelin”, in 1854. Recently it has been established that the decision whether or not an axon is “myelinated” as well as the thickness of the myelin sheath depends on the axonal levels of a particular type of protein of the family of “neuregulins”.
In our group we try to elucidate the molecular mechanisms controlling the axonal myelination. Our goal is to use this information to develop new strategies in the treatment of demyelinating diseases such as multiple sclerosis or Canavan disease in the central nervous system, and Charcot-Marie-Tooth in the peripheral nervous system. We also use this information to try to improve nerve regeneration after traumatic injuries. In order to achieve our goals we use state-of-the-art technologies such us Next-Generation Sequencing of patient’s DNA and genetic modification of mice using both conventional and the CRISPR/CAS9 technology.
Representative Publications
- Schwann cell JUN expression worsens motor performance in an amyotrophic lateral sclerosis mouse model. Sonia Cabeza-Fernández, Rubí Hernández-Rojas, Angeles Casillas-Bajo, Nikiben Patel, Alerie G de la Fuente , Hugo Cabedo , Jose A Gomez-Sanchez Glia. 2024 2024 Aug 16. Online ahead of print. https://doi.org/10.1002/glia.24604
- Glial cell alterations in diabetes-induced neurodegeneration. Llorián-Salvador, M., Cabeza-Fernández, S., Gomez-Sanchez, J.A, de la Fuente A. Cell. Mol. Life Sci. 2024 81: 47 https://doi.org/10.1007/s00018-023-05024-y
- SARM1 detection in myelinating glia: sarm1/Sarm1 is dispensable for PNS and CNS myelination in zebrafish and mice. Fazal SV, Mutschler C, Chen CZ, Turmaine M, Chen CY, Hsueh YP, Ibañez-Grau A, Loreto A, Casillas-Bajo A, Cabedo H, Franklin RJM, Barker RA, Monk KR, Steventon BJ, Coleman MP, Gomez-Sanchez JA, Arthur-Farraj P. Front Cell Neurosci. 2023 17: 1158388 . eCollection 2023. PMID: 370919 https://doi.org/10.3389/fncel.2023.1158388
- A genetic compensatory mechanism regulated by Jun and Mef2d modulates the expression of distinct class IIa Hdacs to ensure peripheral nerve myelination and repair Velasco-Aviles S, Patel N, Casillas-Bajo A, Frutos-Rincón L, Velasco E, Gallar J, Arthur-Farraj P, Gomez-Sanchez JA, Cabedo H eLife 2022 11:e72917 https://doi.org/10.7554/eLife.72917
- Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun Wagstaff LJ, Gomez-Sanchez JA, Fazal SV, Otto GW, Kilpatrick AM, Michael K, Wong LY, Ma KH, Turmaine M, Svaren J, Gordon T, Arthur-Farraj P, Velasco-Aviles S, Cabedo H, Benito C, Mirsky R, Jessen KR eLife 2021 10:e62232 https://doi.org/10.7554/eLife.62232
- Novel EGR2 variant that associates with Charcot-Marie-Tooth disease when combined with lipopolysaccharide-induced TNF-alpha factor T49M polymorphism Blanco-Cantó ME, Patel N, Velasco-Aviles S, Casillas-Bajo A, Salas-Felipe J, García-Escrivá A, Díaz-Marín C, Cabedo H Neurol Genet 2020 6(2):e407 https://doi.org/10.1212/NXG.0000000000000407
- Class IIa HDACs in myelination Velasco-Aviles S, Gomez-Sanchez JA, Cabedo H Aging-US 2018 10(5):853 https://doi.org/10.18632/aging.101443
- Class IIa histone deacetylases link cAMP signaling to the myelin transcriptional program of Schwann cells Gomis-Coloma C, Velasco-Aviles S, Gomez-Sanchez JA, Casillas-Bajo A, Backs J, Cabedo H J Cell Biol 2018 217(4):1249 https://doi.org/10.1083/jcb.201611150
- Schwann cell JUN expression worsens motor performance in an amyotrophic lateral sclerosis mouse model. Sonia Cabeza-Fernández, Rubí Hernández-Rojas, Angeles Casillas-Bajo, Nikiben Patel, Alerie G de la Fuente , Hugo Cabedo , Jose A Gomez-Sanchez Glia. 2024 2024 Aug 16. Online ahead of print. https://doi.org/10.1002/glia.24604
- Glial cell alterations in diabetes-induced neurodegeneration. Llorián-Salvador, M., Cabeza-Fernández, S., Gomez-Sanchez, J.A, de la Fuente A. Cell. Mol. Life Sci. 2024 81: 47 https://doi.org/10.1007/s00018-023-05024-y
- Regulatory T cells limit age-associated retinal inflammation and neurodegeneration. María Llorián-Salvador, Alerie G. de la Fuente, Christopher E. McMurran, Amy Dashwood, James Dooley, Adrian Liston, Rosana Penalva, Yvonne Dombrowski, Alan W. Stitt & Denise C. Fitzgerald. Molecular Neurodegeneration. 2024 19: 32 (2024) https://doi.org/10.1186/s13024-024-00724-w
- SARM1 detection in myelinating glia: sarm1/Sarm1 is dispensable for PNS and CNS myelination in zebrafish and mice. Fazal SV, Mutschler C, Chen CZ, Turmaine M, Chen CY, Hsueh YP, Ibañez-Grau A, Loreto A, Casillas-Bajo A, Cabedo H, Franklin RJM, Barker RA, Monk KR, Steventon BJ, Coleman MP, Gomez-Sanchez JA, Arthur-Farraj P. Front Cell Neurosci. 2023 17: 1158388 . eCollection 2023. PMID: 370919 https://doi.org/10.3389/fncel.2023.1158388
- A genetic compensatory mechanism regulated by Jun and Mef2d modulates the expression of distinct class IIa Hdacs to ensure peripheral nerve myelination and repair Velasco-Aviles S, Patel N, Casillas-Bajo A, Frutos-Rincón L, Velasco E, Gallar J, Arthur-Farraj P, Gomez-Sanchez JA, Cabedo H eLife 2022 11:e72917 https://doi.org/10.7554/eLife.72917
- Emerging Role of HDACs in Regeneration and Ageing in the Peripheral Nervous System: Repair Schwann Cells as Pivotal Targets. Gomez-Sanchez JA, Patel N, Martirena F, Fazal SV, Mutschler C, Cabedo H. Int J Mol Sci. 2022 23(6): art 2996 - Review https://doi.org/10.3390/ijms23062996
- Fasciclin 2 engages EGFR in an auto-stimulatory loop to promote imaginal disc cell proliferation in Drosophila. Velasquez E, Gomez-Sanchez JA, Donier E, Grijota-Martinez C, Cabedo H, Garcia-Alonso L PLoS Genet. 2022 18(6): art. e1010224 https://doi.org/10.1371/journal.pgen.1010224
- Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun Wagstaff LJ, Gomez-Sanchez JA, Fazal SV, Otto GW, Kilpatrick AM, Michael K, Wong LY, Ma KH, Turmaine M, Svaren J, Gordon T, Arthur-Farraj P, Velasco-Aviles S, Cabedo H, Benito C, Mirsky R, Jessen KR eLife 2021 10:e62232 https://doi.org/10.7554/eLife.62232
- Novel EGR2 variant that associates with Charcot-Marie-Tooth disease when combined with lipopolysaccharide-induced TNF-alpha factor T49M polymorphism Blanco-Cantó ME, Patel N, Velasco-Aviles S, Casillas-Bajo A, Salas-Felipe J, García-Escrivá A, Díaz-Marín C, Cabedo H Neurol Genet 2020 6(2):e407 https://doi.org/10.1212/NXG.0000000000000407
- Class IIa HDACs in myelination Velasco-Aviles S, Gomez-Sanchez JA, Cabedo H Aging-US 2018 10(5):853 https://doi.org/10.18632/aging.101443
- Class IIa histone deacetylases link cAMP signaling to the myelin transcriptional program of Schwann cells Gomis-Coloma C, Velasco-Aviles S, Gomez-Sanchez JA, Casillas-Bajo A, Backs J, Cabedo H J Cell Biol 2018 217(4):1249 https://doi.org/10.1083/jcb.201611150
- Epigenetic induction of the Ink4a/Arf locus prevents Schwann cell overproliferation during nerve regeneration and after tumorigenic challenge Gomez-Sanchez JA, Gomis-Coloma C, Morenilla-Palao C, Peiro G, Serra E, Serrano M, Cabedo H Brain 2013 136:2262 https://doi.org/10.1093/brain/awt130

 Español
Español