Nuestra Unidad de Vectores Neurotrópicos está dedicada a la producción de vectores de origen viral para el estudio del sistema nervioso en salud y enfermedad.
En los últimos años, la entrega de herramientas moleculares en las neuronas se ha convertido en un enfoque esencial para comprender los mecanismos que subyacen a la función cerebral y los trastornos cerebrales.
Los virus modificados genéticamente se han convertido en vectores ideales para introducir estas herramientas en las células cerebrales, lo que permite a los neurocientíficos un control sin precedentes sobre las células y los circuitos.
Para facilitar el uso de estas metodologías de vanguardia por parte de nuestros neurocientíficos, la Unidad de Vectores centraliza el proceso de producción y distribución de vectores neurotrópicos.
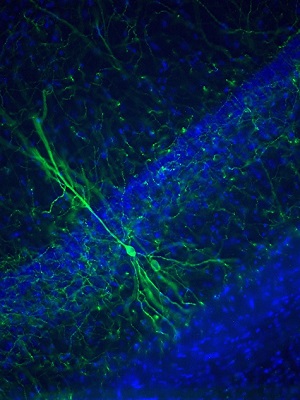
Hippocampal cell infected with a EGFP expressing AAV.(fig.1)
(texto English)
We currently offer custom-made Adeno-associated virus (AVV), Retrovirus (RV) and Lentivirus (LV)-based vector preparations for neuroscience purposes.
AAV specifications:
- Plasmids: The Unit provides plasmids encoding for the most common capsids (1,2,5,8,9,11, PHP.eB, PHP.S, 2 retro, 9p31, ROOT) and helper genes. Because it is important to pick the viral vector that best suits the specific needs, we encourage the users to contact the Unit for advice. Specific gene/construct of interest plasmids should be provided by the user.
- Production: Our AAV vector production method utilizes the triple transfection protocol in HEK293 cells followed by purification using iodixanol gradients, to achieve greater enrichment in infecting viral particles, and a final concentration step. Our titering method is based on Q-PCR.
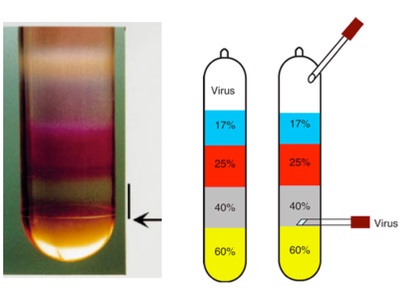
Iodixanol purification (Grieger et al, 2006)
- Safety: AAV vectors are biosafety level 1 and consist of recombinant transgene sequences (e.g., reporter gene) flanked by the AAV inverted terminal repeats. The removal of the majority of viral structural genes renders the vector replication-defective and dependent on an AAV helper virus.
- Handling and storage: All viral vectors are delivered frozen and should be stored at -80° C for long term storage. Vectors can be stored for short periods of time at -20 or +4°C. Whenever possible, vectors should be aliquoted into single use portions to avoid repeated freeze/thaw cycles. Please aliquot in at least 5ul per tube and use low protein binding tubes to avoid loss of virus.
Vectors are ready to use upon reception. If you need to dilute the virus, you may use PBS, but do so ONLY prior to use. Do not dilute unless ready to use.
RV and LV specifications:
- Plasmids: The Unit provides plasmids encoding for the most common envelope glycoproteins (VSVG and Rabies Glycoprotein) and helper genes (gag, pol). Because it is important to pick the viral vector that best suits the specific needs, we encourage the users to contact the Unit for advice. Specific gene/construct of interest plasmids should be provided by the user.
- Production: Our RV and LV vectors production method utilizes the triple transfection protocol in HEK293-T cells followed by filtration and ultracentrifugation to achieve high concentrations compatible with their use in vivo. Our titering method is based on Q-RT-PCR.
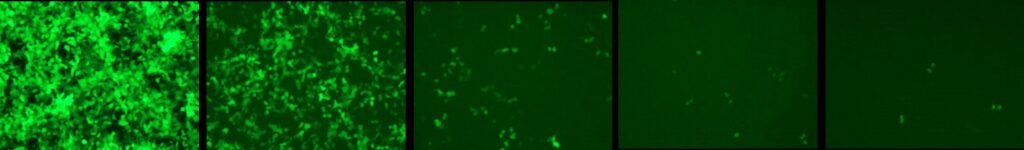
HEK293 cells infected with serial dilutions of a EGFP expressing LV
- Safety: RV and LV vectors are biosafety level 2 and consist of recombinant transgene sequences (e.g., reporter gene) flanked by the RV or LV long terminal repeats (LTR). The removal of the majority of viral structural genes renders the vector replication-defective. RV and LV vector sequences integrate randomly on the host genome.
- Handling and storage: All viral vectors are delivered frozen and should be stored at -80° C for long term storage. Vectors can be stored for short periods of time at -20. Whenever possible, vectors should be aliquoted into single use portions to avoid repeated freeze/thaw cycles. Please aliquot in at least 5ul per tube and use low protein binding tubes to avoid loss of virus.
Vectors are ready to use upon reception. If you need to dilute the virus, you may use PBS, but do so ONLY prior to use. Do not dilute unless ready to use.
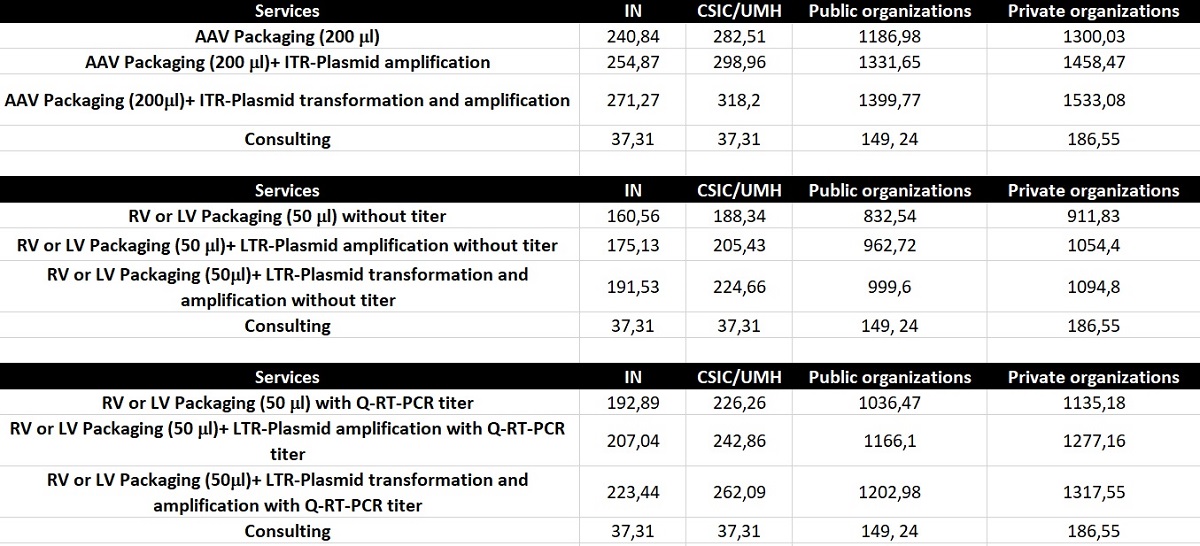
El coste de cada preparación dependerá de las necesidades específicas del usuario. Las tarifas básicas se enumeran a continuación. Póngase en contacto con la Unidad para obtener una tarifa personalizada.
email: vectores@umh.es
Table 1. Price list (€) 2025
El espacio de la unidad de Vectores Neurotrópicos incluye suites separadas para el trabajo de ADN y virus asignados respectivamente en:
- Laboratorio 203.
- Laboratorio de bioseguridad (NCB2)

La Unidad cuenta con todo el equipo básico necesario para la producción de vectores virales (incluida una instalación BSL-2 equipada con campanas de cultivo de tejidos e incubadoras, centrífugas de mesa, ultracentrífugas) y titulación (laboratorio de biología molecular, máquina Quant-Studio PCR).

 Fotos: Instituto de Neurociencias
Fotos: Instituto de Neurociencias
Para comunicaciones generales, consultas sobre nuestros servicios, pedidos, entrenamiento y formación, protocolos, etc… por favor, contáctenos: vectores@umh.es

 English
English

