Lines of investigation
The main questions we studied are how growth is regulated at the organ, between organs, and at the organism level, and how the disruption of these regulations leads to cancer. We leverage the powerful genetic tools in the fruit fly, Drosophila melanogaster, to address these questions. The precision and robustness of such growth control are illustrated by bilateral symmetry. Think, for example, of how a human face, or the wings of an insect, are perfectly matched in size and shape, even though each part grows separately. This precision is striking when considering the noisiness of gene expression, unavoidable environmental perturbations, errors, and mutations. We have found that an individual’s degree of bilateral symmetry or asymmetry reflects the vulnerability of development and growth processes to such perturbations and the shortage of mechanisms that buffer such variation. Our lab discovered that developmental buffering involves organ-organ and organ-brain communication mediated by a hormone of the relaxin family (Ilp8) and its receptor Lgr3 expressed in the CNS. In addition, to the high-order control of growth, cell-cell communication and cancer cell-host communication are important in both the prevention and initiation and progression of cancer. This communication is mediated by hormones and signaling pathways such as the Notch, PI3K/AKT/PTEN, and JAK/SAT pathways. The main findings in this area are the demonstration that in response to fat levels, a neuroendocrine axis is activated and matures that activates sexual maturation in the fly Drosophila melanogaster and that this axis causes obesity when inactive, but can be rescued with the hormone human Leptin, demonstrating conservation of processes. Furthermore, we have reported that hormones produced by the brain can remodel the size of adult organs such as the gut to sustain the high energetic demands of reproduction.
Representative Publications
- Body-fat sensor triggers ribosome maturation in the steroidogenic gland to initiate sexual maturation in Drosophila Juarez-Carreño S, Vallejo DM, Carranza-Valencia J, Palomino-Schätzlein M, Ramon-Cañellas P, Santoro R, de Hartog E, Ferres-Marco D, Romero A, Peterson HP, Ballesta-Illan E, Pineda-Lucena A, Dominguez M, Morante J Cell Rep 2021 37(2):109830 https://doi.org/10.1016/j.celrep.2021.109830
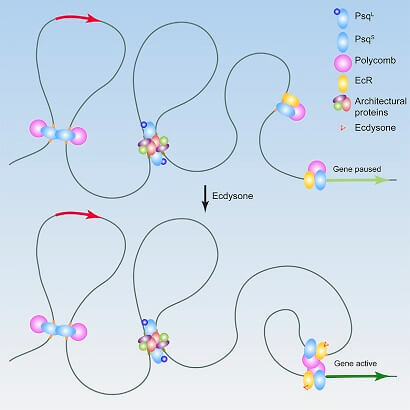
- Ecdysone-Induced 3D Chromatin Reorganization Involves Active Enhancers Bound by Pipsqueak and Polycomb Gutierrez-Perez I, Rowley MJ, Lyu X, Valadez-Graham V, Vallejo DM, Ballesta-Illan E, Lopez-Atalaya JP, Kremsky I, Caparros E, Corces VG, Dominguez M Cell Rep 2019 28(10):2715 https://doi.org/10.1016/j.celrep.2019.07.096
- Notch and EGFR regulate apoptosis in progenitor cells to ensure gut homeostasis in Drosophila Reiff T, Antonello ZA, Ballesta-Illán E, Mira L, Sala S, Navarro M, Martinez LM, Dominguez M EMBO Journal 2019 38(21):e101346 https://doi.org/10.15252/embj.2018101346
- PI3K/Akt Cooperates with Oncogenic Notch by Inducing Nitric Oxide-Dependent Inflammation Villegas SN, Gombos R, Garcia-Lopez L, Gutierrez-Perez I, Garcia-Castillo J, Vallejo DM, Da Ros VG, Ballesta-Illan E, Mihaly J, Dominguez M Cell Rep 2018 22(10):2541 https://doi.org/10.1016/j.celrep.2018.02.049
- A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3 Vallejo DM, Juarez-Carreño S, Bolivar J, Morante J, Dominguez M Science 2015 350(6262):aac6767 https://doi.org/10.1126/science.aac6767
- Conserved miR-8/miR-200 Defines a Glial Niche that Controls Neuroepithelial Expansion and Neuroblast Transition Morante J, Vallejo DM, Desplan C, Dominguez M Dev Cell 2013 27(2):174 https://doi.org/10.1016/j.devcel.2013.09.018
- Body-fat sensor triggers ribosome maturation in the steroidogenic gland to initiate sexual maturation in Drosophila Juarez-Carreño S, Vallejo DM, Carranza-Valencia J, Palomino-Schätzlein M, Ramon-Cañellas P, Santoro R, de Hartog E, Ferres-Marco D, Romero A, Peterson HP, Ballesta-Illan E, Pineda-Lucena A, Dominguez M, Morante J Cell Rep 2021 37(2):109830 https://doi.org/10.1016/j.celrep.2021.109830
- A Blueprint for Cancer-Related Inflammation and Host Innate Immunity García-López L, Adrados I, Ferres-Marco D, Dominguez M Cells 2021 10(11):3211 https://doi.org/10.3390/cells10113211
- Ecdysone-Induced 3D Chromatin Reorganization Involves Active Enhancers Bound by Pipsqueak and Polycomb Gutierrez-Perez I, Rowley MJ, Lyu X, Valadez-Graham V, Vallejo DM, Ballesta-Illan E, Lopez-Atalaya JP, Kremsky I, Caparros E, Corces VG, Dominguez M Cell Rep 2019 28(10):2715 https://doi.org/10.1016/j.celrep.2019.07.096
- Notch and EGFR regulate apoptosis in progenitor cells to ensure gut homeostasis in Drosophila Reiff T, Antonello ZA, Ballesta-Illán E, Mira L, Sala S, Navarro M, Martinez LM, Dominguez M EMBO Journal 2019 38(21):e101346 https://doi.org/10.15252/embj.2018101346
- One hundred years of Drosophila cancer research: no longer in solitude Villegas SN DMM Disease Models and Mechanisms 2019 12(4):UNSP dmm039032 https://doi.org/10.1242/dmm.039032
- Using Drosophila Models and Tools to Understand the Mechanisms of Novel Human Cancer Driver Gene Function Villegas SN, Ferres-Marco D, Domínguez M Adv Exp Med Biol 2019 1167:15 https://doi.org/10.1007/978-3-030-23629-8_2
- Histone variant H2A.Z deposition and acetylation directs the canonical Notch signaling response Giaimo BD, Ferrante F, Vallejo DM, Hein K, Gutierrez-Perez I, Nist A, Stiewe T, Mittler G, Herold S, Zimmermann T, Bartkuhn M, Schwarz P, Oswald F, Dominguez M, Borggrefe T Nucleic Acids Res 2018 46(16):8197 https://doi.org/10.1093/nar/gky551
- PI3K/Akt Cooperates with Oncogenic Notch by Inducing Nitric Oxide-Dependent Inflammation Villegas SN, Gombos R, Garcia-Lopez L, Gutierrez-Perez I, Garcia-Castillo J, Vallejo DM, Da Ros VG, Ballesta-Illan E, Mihaly J, Dominguez M Cell Rep 2018 22(10):2541 https://doi.org/10.1016/j.celrep.2018.02.049
- Systemic signalling and local effectors in developmental stability, body symmetry, and size Juarez-Carreno S, Morante J, Dominguez M Cell Stress 2018 2(12):340 https://doi.org/10.15698/cst2018.12.167
- A phospho-dependent mechanism involving NCoR and KMT2D controls a permissive chromatin state at Notch target genes Oswald F, Rodriguez P, Giaimo BD, Antonello ZA, Mira L, Mittler G, Thiel VN, Collins KJ, Tabaja N, Cizelsky W, Rothe M, Kühl SJ, Kühl M, Ferrante F, Hein K, Kovall RA, Dominguez M, Borggrefe T Nucleic Acids Res 2016 44(10):4703 https://doi.org/10.1093/nar/gkw105
- Endocrine remodelling of the adult intestine sustains reproduction in Drosophila Reiff T, Jacobson J, Cognigni P, Antonello Z, Ballesta E, Tan KJ, Yew JY, Dominguez M, Miguel-Aliaga I eLife 2015 4:e06930 https://doi.org/10.7554/eLife.06930
- Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8-Escargot switch Antonello ZA, Reiff T, Ballesta-Illan E, Dominguez M EMBO J 2015 34(15):2025 https://doi.org/10.15252/embj.201591517
- Mesenchymal to epithelial transition during tissue homeostasis and regeneration: Patching up the Drosophila midgut epithelium Antonello ZA, Reiff T, Dominguez M Fly 2015 9(3):132 https://doi.org/10.1080/19336934.2016.1140709
- acal is a long non-coding RNA in JNK signaling in epithelial shape changes during drosophila dorsal closure Rios-Barrera LD, Gutierrez-Perez I, Dominguez M, Riesgo-Escovar JR PLoS Genet 2015 11(2):e1004927 https://doi.org/10.1371/journal.pgen.1004927
- A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3 Vallejo DM, Juarez-Carreño S, Bolivar J, Morante J, Dominguez M Science 2015 350(6262):aac6767 https://doi.org/10.1126/science.aac6767
- Oncogenic programmes and Notch activity: An ‘organized crime’? Dominguez M Semin Cell Dev Biol 2014 28:78 https://doi.org/10.1016/j.semcdb.2014.04.012
- Chromatin-Bound I?B-alpha Regulates a Subset of Polycomb Target Genes in Differentiation and Cancer Mulero MC, Ferres-Marco D, Islam A, Margalef P, Pecoraro M, Toll A, Drechsel N, Charneco C, Davis S, Bellora N, Gallardo F, Lopez-Arribiliaga E, Asensio-Juan E, Rodilla V, Gonzalez J, Iglesias M, Shih V, Mar Alba M, Di Croce L, Hoffmann A, Miyamoto S, Villa-Freixa J, Lopez-Bigas N, Keyes WM, Dominguez M, Bigas A, Espinosa L Cancer Cell 2013 24(2):151 https://doi.org/10.1016/j.ccr.2013.06.003
- Conserved miR-8/miR-200 Defines a Glial Niche that Controls Neuroepithelial Expansion and Neuroblast Transition Morante J, Vallejo DM, Desplan C, Dominguez M Dev Cell 2013 27(2):174 https://doi.org/10.1016/j.devcel.2013.09.018
- Dampening the Signals Transduced through Hedgehog via MicroRNA miR-7 Facilitates Notch-Induced Tumourigenesis Da Ros VG, Gutierrez-Perez I, Ferres-Marco D, Dominguez M PLoS Biol 2013 11(5):e1001554 https://doi.org/10.1371/journal.pbio.1001554
- Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia Ntziachristos P,Tsirigos A, Vlierberghe PV, Nedjic J, Trimarchi T, Flaherty MS, Ferres-Marco D, da Ros V, Tang Z, Siegle T, Dominguez M, Ferrando A, Aifantis I Nat Med 2012 18(2):298 https://doi.org/10.1038/nm.2651
- Imaginal Discs Secrete Insulin-Like Peptide 8 to Mediate Plasticity of Growth and Maturation Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M Science 2012 336(6081):579 https://doi.org/10.1126/science.1216735
- Intron retention in the Drosophila melanogaster Rieske iron sulphur protein gene generated a new protein Gontijo AM, Miguela V, Whiting MF, Woodruff RC, Dominguez M Nat Commun 2011 2:323 https://doi.org/10.1038/ncomms1328
- Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells Vallejo DM, Caparros E, Dominguez M EMBO J 2011 30(4):756 https://doi.org/10.1038/emboj.2010.358
- Histone demethylase KDM5A is an integral part of the core Notch-RBP-J repressor complex Liefke R, Oswald F, Alvarado C, Ferres-Marco D, Mittler F, Rodriguez P, Dominguez M, Borggrefe T Genes Dev 2010 24(6):590 https://doi.org/10.1101/gad.563210

 Español
Español