The Instituto de Neurociencias (IN) Microscopy Facility is a platform for microscopy and image analysis that provides services and training to both IN and external users. This core facility includes a set of state-of-the-art equipment that allows to perform a great variety of techniques including confocal microscopy, multiphoton, light-sheet (in vivo and clarified) or super-resolution microscopy (Airyscan, SR-SIM, PALM / dSTORM). Images and videos from fixed samples, living tissues, cell cultures, slices or even intact animals can be acquired. The service also counts with high-performance workstations and software packages for image processing and analysis.
Technological Offer (file PDF)





Services offered
- Training and technical assistance for the use of all the devices and equipment provided in the Facility.
- Coaching/guidance for experimental design.
- Training and assistance in the processing and analysis of images using the Facility’s commercial and freely available software.
- Guidelines elaboration and assistance in the writing of materials and methods for publications.
- Maintenance and improvement of equipment and the entire infrastructure of the unit.
- Guidance in the acquisition of microscopy equipment.
- Equipment and device maintenance.
- Workshop organization and displays related to image acquisition, processing and analysis, as well as new technologies.
- Preparation of documentation for the acquisition of new equipment for the Microscopy Service.
- Participation in outreach activities.
TRAINING REQUEST
Fill in the following form to make a training request to the Microscopy Facility:
LEICA TECHNICAL SERVICE REQUEST
Fill in the following form to make a request for Leica Technical Service:
FACILITY REQUESTS
General communication, questions and appointment inquiries will be set up by email: microscopia@umh.es
Multiphoton Microscopes (Lab 020)

Vertical Multiphoton Microscope Leica SP5 allows to perform in vivo and in vitro imaging experiments combined with electrophysiological recordings. Includes an infrared laser MaiTai DeepSee modulable from 690nm to 1040nm for multiphoton acquisition as well as highly sensitive external hybrid detectors for multiphoton acquisition. It also has a multiline argon laser (458, 476, 488, 496 and 515nm), a 561nm diode, and a 633nm laser for confocal acquisition. Often used objectives are the dry 5x, and water-immersed and broad working distances 25x and 63x.
Confocal Microscopes
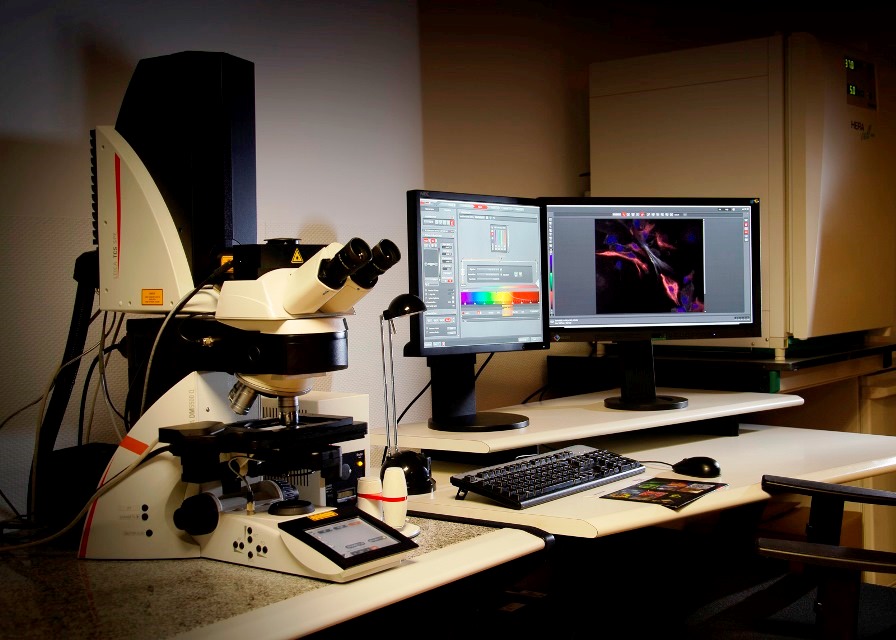
Vertical Confocal Microscope Leica SPEII (Lab 021) equipped with a programmable sample holder for multilocation acquisition. It has four lasers (405nm, 488nm, 561nm and 635nm) and four objectives (dry 10x and 20x, oil-immersion 40x and 63x).
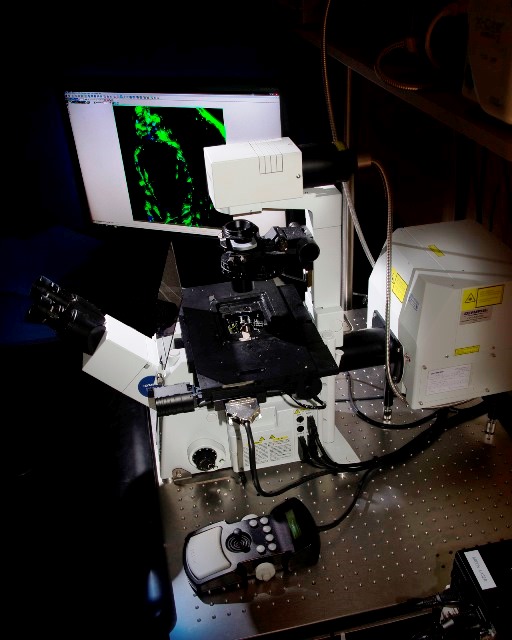
Inverted Confocal Microscope Olympus FV1200 (Lab 021) with a programmable XY sample holder for experiments requiring multilocation and mosaic acquisitions. It has a violet laser (405nm), a multiline argon laser (473, 488 and 515nm), a 559nm laser, and a 635nm laser. It has dry 10x and 20x objectives, as well as oil-immersed 20x, 40x and 60x objectives.
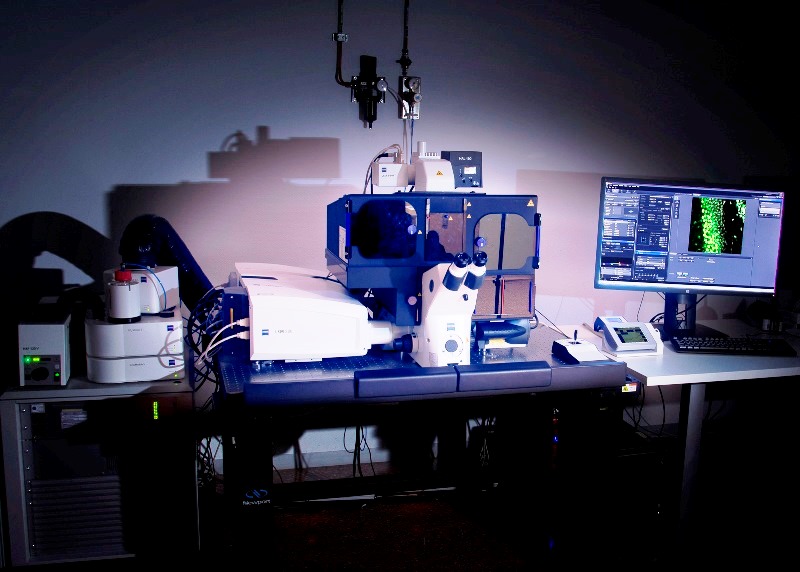
Super-resolution Inverted Confocal Microscope Zeiss LSM 880-Airyscan Elyra PS.1 (Lab 021) with temperature control and CO2 for multidimensional acquisition on fixed biological tissue and living biological samples. It allows confocal image acquisition and also Airyscan (140nm), structured illumination SIM (100 nm) and PALM/dSTORM (20-30nm) super-resolution acquisitions. It is equipped with two lasers: for confocal microscopy (405nm, 458, 488 and 514nm, 561 and 634nm) and super-resolution microscopy (405nm, 488nm, 561nm and 640nm). Objectives: dry 10x, multi-immersion 25x, water-immersion 40x and 63x, oil-immersion 63x and 100 x.

Dragonfly Spinning Disk Confocal microscope: Spinning disk confocal microscope for real-time imaging of dynamic processes. It consists of an incubation system, motorized stage, two Andor Zyla 4.2 cameras for simultaneous dual acquisitions and four laser lines (405, 488, 561 and 637nm). Objectives: 10x and 20x dry, 20x multi-immersion (oil, glycerin and water), 25x LWD water immersion, as well as 40x and 60x oil.
Light-sheet Microscopes
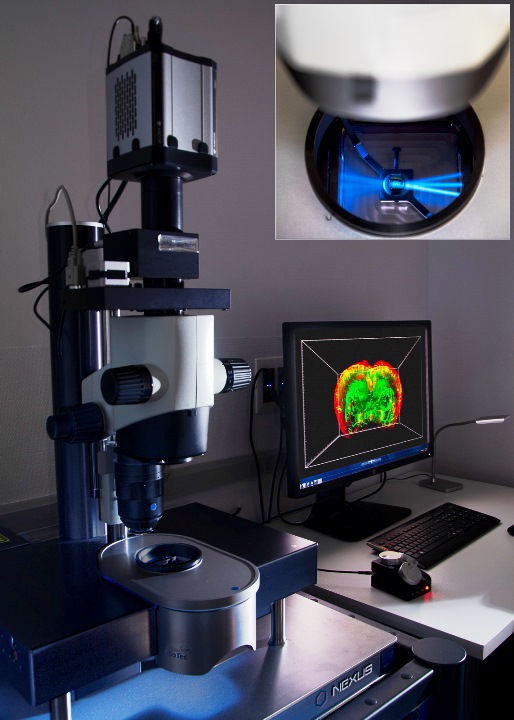
Light-sheet UltramicroscopeII LaVisionBiotec for clarified samples. It has lasers to acquire 488nm, 561nm and 635nm. Acquisition objective is 2x with optical zoom from 0.63x to 6.3x, allowing dynamic magnification from 1.26x to 12.6x..
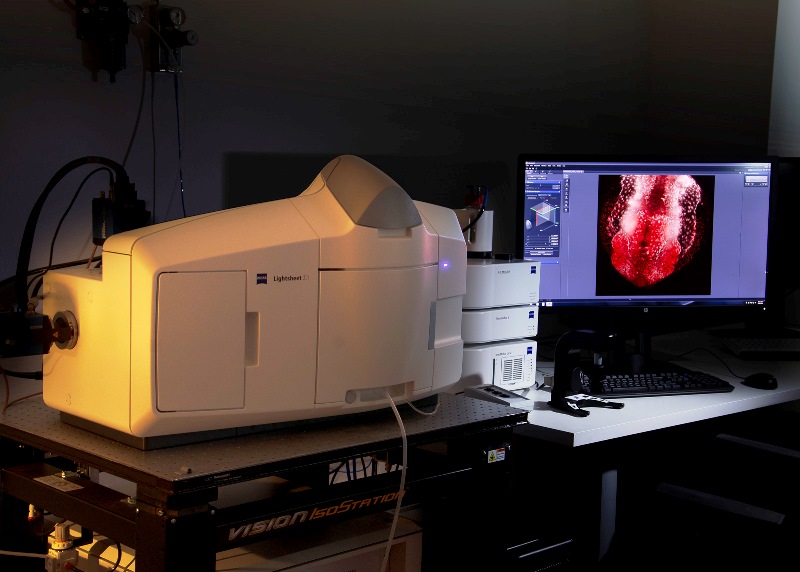
Light-sheet Microscope Z.1 Zeiss for semi-transparent living samples, it allows acquisition of 360º images. It is equipped with 405nm, 488nm, 561nm and 635nm lasers. It has three objectives: a dry 5x and two water-immersion (10x and 20x).
Widefield Microscopes
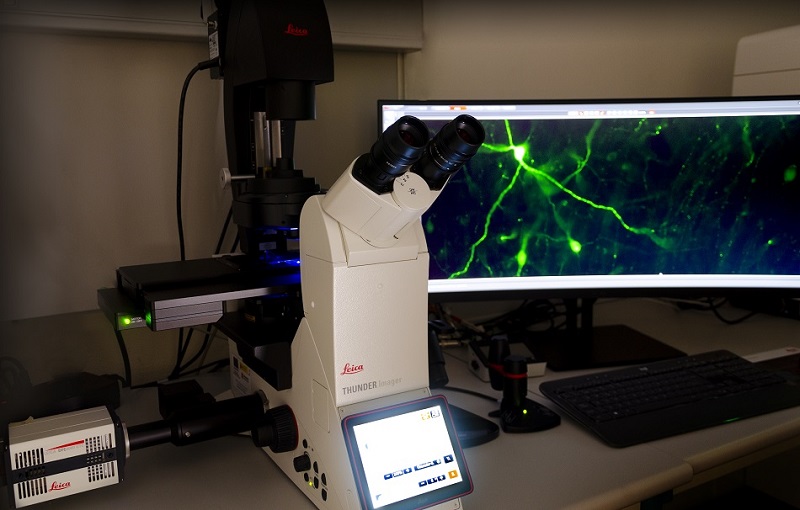
Fully automated widefield microscope for optical sectioning image acquisition by Computational Clearing in fixed or live samples. LED illumination with 8 excitation lines from UV to far red. 5x/0.12, 10x/0.32, and 20x/0.80 dry objectives, plus 40x/1.30 and 63x/1.40 oil.
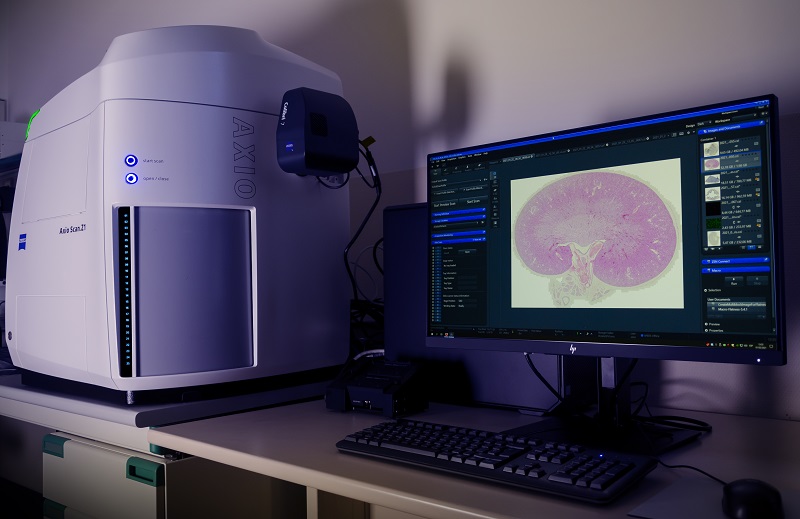
Fully automated scanner for fluorescent or brightfield samples, with an up to 100 slides capacity. LED Illumination with 7 excitation lines from UV to far red. 5x/0.25, 10x/0.45, 20x/0.8 and 40x/0.95 dry objectives

Neurolucida System (Lab 021) for brain tissue and neuron reconstruction and analysis. Based on a Leica DM4000 fluorescence/transmitted light microscope. It is equipped with fluorescence filters for DAPI, GFP, Cy3 and Cy5. It has dry 2.5x, 10x, 20x and 40x objectives, and an oil-immersion 63x objective. Detection can be performed with a color camera (MBF CX9000) or a monochrome camera (QImaging).

Vertical Leica DM5000B Microscope, for fluorescence and brightfield illumination of fixed samples. It has filter cubes for DAPI, GFP and Cy3 and dry objectives of 2.5x, 5x, 10x, 20x and 40x.
Leica MZFLIII Stereomicroscope
Leica MZFLIII stereomicroscope (Lab 021)
Workstations
HIVE high-performance workstation
It is a high-end centralized workstation for image processing and high-performance/GPU computing, optimal for demanding applications and multi-user configurations. It contains ZEISS arivis Pro, Huygens Professional, FIJI, QuPath, Zen Blue (Zeiss) and Las X (Leica) software.
Super-resolution (SR) Workstation
Zen: https://www.zeiss.com/microscopy/int/products/microscope-software/zen.html
Equipped with ZEN Black, ZEN Blue, Huygens Professional, Leica LAS X Core Widefield, Imaris Viewer, ImageJ/Fiji.
Lightsheet (LS) Workstation
Equipped with: ZEN Black, ZEN Blue 3.3 Lite, Leica LAS X Core Widefield, Imaris Viewer , ImageJ/Fiji, Huygens Professional.
Imaris I Workstation
Equipped with: Imaris, AutoQuant X3, Huygens Professional, ImageJ/Fiji, Olympus FV10-ASW Viewer, Leica LAS X Core, ZEN Blue 3.3 Lite de Zeiss, Terrastitcher ImspectorPro de LaVisionBiotec.
Imaris II Workstation
Equipped with: Imaris, Imaris Stitcher, ImageJ/Fiji 64 bit, Olympus FV10-ASW Viewer, Leica LAS X Core, ZEN Blue 3.3 Lite de Zeiss, ClearMap, Huygens Core HRM.
Workstation
ImageJ/Fiji, Neurolúcida Explorer, Olympus FV10ASW Viewer, ZEN Blue 3.3 Lite y Leica LAS X Core.
Network Access & Storage Server (NAS)
For temporary storage of files generated on the facility equipment that require further processing with Imaging Facility scientific software.
Rules
View the document “Guidelines of the Microscopy Facility” found in the ARCHIVES section for a more complete description of the facility operation.
General operational considerations of the Microscopy Facility:
-
General communication, questions, appointment inquiries, and training requests will be established by email to: microscopia@umh.es.
-
The Principal Investigator is ultimately responsible for the correct use of the Facility’s equipment by any member of his/her team.
-
All research personnel affiliated with the IN and those visiting researchers who are going to stay at the IN for more than 6 months will have the status of Microscopy Facility user (with the exception of those who can prove that they are experts in microscopy techniques).
-
Master’s or degree students may use the equipment only under the company of a supervisor.
-
It is the obligation of each user to know the operating rules of the Microscopy Service, as well as the specific instructions for handling each equipment used. These are included in the document “Imaging Facility Guidelines”.
-
It is mandatory for new users to receive the corresponding training by the Facility’s personnel before using any equipment. Moreover, during the first sessions, they should be supervised by the Facility’s personnel or an experienced user.
-
Training requests will be made through the training request form located in the “Services” section of our website, once the new user:
– Has their own prepared samples and sufficient specific knowledge about them.
– Has clear specific questions to answer with the facility equipment.
– Is going to use the equipment imminently. -
The reserved user is responsible for the state of the equipment during that period.
-
The last booked user of the day is responsible for turning off the microscope and all its components.
-
The working area should be kept clean, particularly after finalizing the session.
-
If you have uncertainties regarding the use of any equipment, no matter how simple may seem, ask for help from the personnel without any hesitation.
-
It is forbidden to browse Internet, software installation/deletion or the use of external storage devices in any Facility’s computer.
-
It is forbidden to store data on the computers of the facility. Users will be responsible for transferring their images once acquired or processes, as well as removing them from their user folder. An automatic file deletion instruction is programmed in all Microscopy Facility equipment. Files older than 30 days since their acquisition will be deleted from the computer and cannot be restored.
-
Users should request their subscription to the mailing list in.microscopia@listas.umh.es through the website https://listas.umh.es/sympa/info/in.microscopia. Communications regarding the Imaging Facility will be made through this mailing list.
-
Microscopes and workstations will be always booked through the web: http://in.umh-csic.es/ > Acceso usuarios > Reservas. All the equipment and software offered by the Imaging Facility begin with the letter “SM”.
-
Users are required to start and end their reservation on time so as not to interfere with the work of other colleagues.
-
Reservations not used during the first 10 minutes will be considered deserted.
-
Although the reservation system allows cancellations of bookings at any time, cancellations on the current day are not allowed and will be penalized if they are not duly justified as follows:
– Users who cancel an appointment reserved electronically on the current day will be penalized with two weeks without access to the service equipment.
– Users who do not use or release a booking via telematics will not be able to use the service equipment for the following four weeks.
– In case of recidivism by the user, use of the Service will be prohibited for three months
Reservations for all the facility equipment will be made through the intranet of the Institute of Neurosciences
Rates
The Microscopy Facility is free for the Instituto de Neurociencias members. If you do not belong to our Institute but would like to collaborate with one of our research teams to use our knowledge and technology in any of your projects, please contact us by email: microscopia@umh.es
To consult our rates for external users, please access the following links of the Technical Support Service for Teaching and Research (Satdi) of the UMH:
“Fluorescent and Confocal Microscopy”:
https://satdi.umh.es/instrumentacioncientifica/servicios_ofertados/microscopia-confocal/
How to use the Satdi service:
https://satdi.umh.es/instrumentacioncientifica/
How to make a service use request:
https://satdi.umh.es/instrumentacioncientifica/formularios/formulario-peticion/
Location
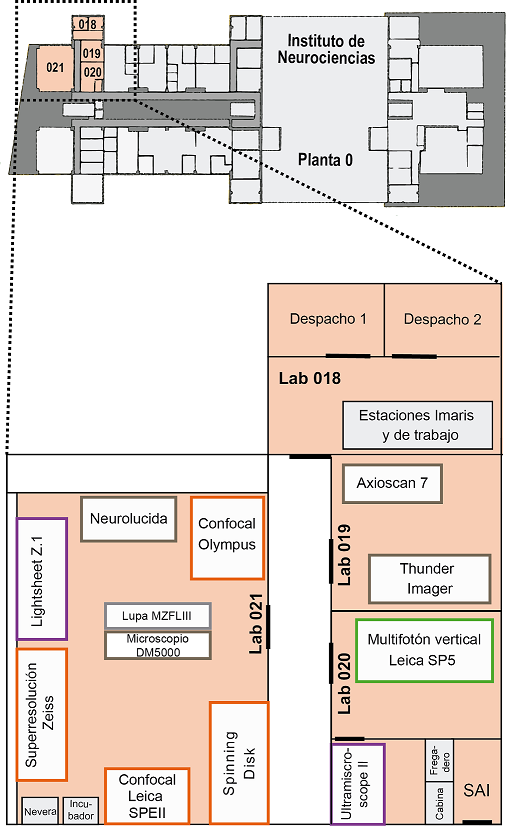
The Microscopy Facility is located on the ground floor, south corner of the IN and organized as follows:
Room 018
- Office 1: Giovanna Expósito
- Office 2: Almudena Íñigo
- Imaris I
- Imaris II
- Workstation
Room 019
- Inverted Microscope Leica Thunder Imager
- Slide scanner Zeiss AxioScan 7
Room 020
- Vertical Multiphoton Microscope Leica SP5
- Inverted Multiphoton Microscope Leica SP2
- Light-sheet Ultramicroscope II LaVisionBiotec
Room 021
- Vertical Confocal Microscope Leica SPEII
- Inverted Confocal Microscope Olympus FV1200
- Inverted Super-resolution Confocal Microscope Zeiss LSM 880-Airyscan Elyra PS.1
- Workstation Super-resolution
- Light-sheetZ.1 Microscope Zeiss
- Workstation Light-sheet in vivo
- Neurolucida System
- Leica MZFLIII fluorescence stereoscope
- Vertical Leica DM5000B Microscope
- Confocal spinning disk Dragonfly
Software packages available at the Facility:
- Imaris (Bitplane): software for visualization, processing, analysis and multichannel image export in 2D, 3D and 4D.
http://www.bitplane.com/ - Zeiss arivis Pro: software for visualization, processing, analysis and multichannel image export in 2D, 3D and 4D.https://www.arivis.com/products/pro
- Neurolucida: http://www.mbfbioscience.com/neurolucida
- Strereo Investigator: https://www.mbfbioscience.com/stereo-investigator
- Huygens Profesional: https://svi.nl/Huygens-Software
- AutoQuant X3: deconvolution software.http://www.mediacy.com/autoquantx
Free softwares for image visualization and analysis:
- ImageJ: free software for processing and analysis of multidimensional images.https://imagej.nih.gov/ij/
- Fiji: same as ImageJ, which includes a complete processing package for scientific image analysis.https://imagej.net/Fiji
- ClearMap: free software crated for the image analysis of iDISCO+ samples. It allows the detection of 3D objects and brain nuclei localization.https://idisco.info/clearmap-2/
- Zeiss: https://www.zeiss.com/microscopy/int/products/microscope-software/zen-lite.html
- Leica: https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/downloads/
Tutorials:
- Imaris: http://www.bitplane.com/learning
- Zeiss arivis Pro: https://kb.arivis.com/arivis-vision4d-video-tutorials-compendium
- ImageJ/Fiji: http://imagej.net/Category:Tutorials#Tutorials_for_new_users
- Neurolucida: http://www.mbfbioscience.com/webinars
- Stereology: https://www.stereology.info
- Optic microscopy: http://zeiss-campus.magnet.fsu.edu/
- Light-sheet microscopy: https://www.youtube.com/watch?v=vKHQudCKVGc
- Huygens Professional: https://svi.nl/tiki-index.php?page=IntroductionMovies
- LAS X Thunder: https://youtu.be/36HqrFyy0fQ
- LAS X Navigator: https://youtu.be/iqMgOmjslf8
- Bioimage analysis: https://bioimagebook.github.io/README.html
Tissue clearing techniques:
- https://idisco.info/
- https://www.lavisionbiotec.com/products/UltraMicroscope/scanner-options.html
- http://clarityresourcecenter.org/
- https://transparent-human-embryo.com/
Ethics in digital image processing:
Spectrum visualization:
» Microscopia de alta resolución automatizada y apoyo a manejo de datos de imagen de alta densidad para Unidad de Imagen EQC2019-006023-P. FEDER/Ministerio de Ciencia e Innovación – Agencia Estatal de Investigación.
» Actualización del Servicio de Imagen celular ECQ_2018_004690_P. FEDER/Ministerio de Ciencia e Innovación Agencia Estatal de Investigación, cofinanciado con el proyecto Severo Ochoa SEV-2017-0732.
» Actualización de Servicio de Imagen Celular CSIC15-EE-3246 FEDER/Ministerio de Economía, Industria y Competitividad, cofinanciado por el proyecto Severo Ochoa SEV-2013-0317.






Scientific Director:
Ana Carmena de la Cruz
acarmena@umh.es »
+34 965 91 92 30

Técnico superior especializado OPIS CSIC:
Giovanna Expósito Romero
gexposito@umh.es »
+34 965 91 92 66
Técnico superior especializado OPIS CSIC:
Aida Giner de Gracia
aginer@umh.es
+34 965 91 92 66
General communication, questions, appointment inquiries, and training requests will be set up by email to: microscopia@umh.es
Microscopy Facility
Instituto de Neurociencias
Universidad Miguel Hernández – CSIC
Campus de San Juan
Sant Joan d’Alacant
Alicante | España
Tel. + 34 965 23 37 00
(ext. service 9266)
Solo visible para miembros.
Acceder

 Español
Español