Intron detention tightly regulates the stemness/differentiation switch in the adult neurogenic niche.
A study from the Institute for Neurosciences CSIC-UMH discovers the mechanism that avoids conflicts in the activity of brain stem cells.
– This finding, published in Nature Communications, represents another step in understanding how brain stem cells work, in their capacity to produce new neurons.
– Brain stem cells express both genes that maintain their identity and genes specific to the neurons they produce.
(Photo: Ángela Nieto, and Joan Galcerán. IN-CSIC-UMH)
Researchers from the Cell Plasticity in Development and Disease laboratory, led by Ángela Nieto at the Institute of Neurosciences (IN), a joint center of the Spanish National Research Council (CSIC) and the Miguel Hernández University (UMH) of Elche, have discovered the mechanism which allows adult brain stem cells to express genes that maintain their identity and those for neuronal differentiation without conflicts in cellular activity. Furthermore, this mechanism enables stem cells to be prepared to respond to differentiation signals readily.
All cells in an organism have the same genes, but the difference between them is in the genes they express and those they do not. This is known as cellular identity and will determine the functions of the cells throughout their life. The case of brain stem cells is interesting because they express the genes that keep them as stem cells, and also other genes that are specific to the neurons they produce.
Until now, it was unknown how brain stem cells managed to express both types of genes without encountering decision conflicts, where the cell faces uncertainty about whether to become a neuron or maintain its stem cell state. However, the results of this study, recently published in Nature Communications, have revealed the mechanism that prevents differentiation genes from being translated in stem cells. This discovery resolves the supposed problem of decision conflicts.
In explaining the process, Ainara González-Iglesias, first author of the article, highlights: “When genes are transcribed, they generate messenger RNAs that are then translated into proteins, the effectors of cellular functions. For this process to occur, these messengers must leave the nucleus of the cell and go to the cytoplasm to be translated”. The experts discovered that the key lies in the messenger RNAs of the stem cell genes leaving the nucleus to be translated into proteins, while those of the neuronal genes were retained in the nucleus. “For this reason, the cells continued to maintain their status as stem cells”, explains the researcher.
A two-way switch
When stem cells need to differentiate into neurons, the mechanism operates similarly. Nieto explains: “In this case, it is the messengers of the stem cell maintenance genes that are retained in the nucleus preventing their translation into proteins”. Despite both types of genes being expressed continuously, the researcher emphasizes that “the messengers of genes not required for immediate function are always retained and not translated. This mechanism not only resolves decision conflicts within cells but also primes the cellular machinery for immediate differentiation upon receiving the appropriate signal”.
Stem cells possess the remarkable ability to regenerate tissues. Although the extent of their contribution to regeneration in the human adult brain remains uncertain, Nieto emphasizes the fundamental role of this. Without it, premature neuronal differentiation could occur, potentially disrupting the proper functioning of the nervous system.
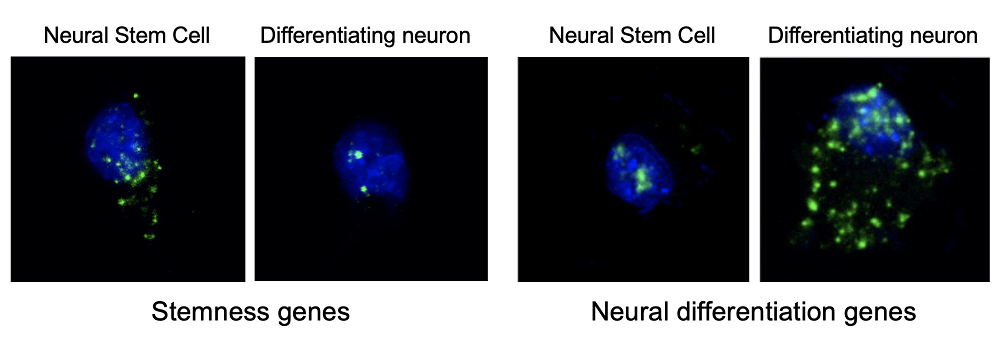
“Stem cells and cells in the process of differentiation into neurons (nuclei in blue and messenger RNA in green). In stem cells, the gene messengers associated with their functions leave the nucleus, while they are retained in the cells in the process of transformation into neurons. The opposite occurs with genes whose function is associated with neuronal differentiation.”
National collaboration
This research was conducted in collaboration with the laboratory led by Isabel Fariñas, an expert in stem cells, at the Institute of Biotechnology and Biomedicine of the University of Valencia, and the laboratory led by Juan Valcárcel, an expert in RNA processing mechanisms, at the Center for Genomic Regulation in Barcelona. The study is focused on the subventricular zone of the adult mouse brain, which harbours a large population of stem cells.
Through this investigation, they confirmed that the retention mechanism in the nucleus is associated with the lack of an RNA modification called methylation. This modification triggers the elimination of introns, which are fragments of messenger RNA that must be removed for the proper nuclear export and translation of the messengers.
The experts meticulously observed how the messenger RNA was retained in the nucleus using in situ hybridization, a technique that visualizes messenger RNA in tissues. This technique, widely employed in Nieto´s laboratory at the IN, proved instrumental in identifying the mechanism. Ainara González-Iglesias highlights the importance of this technique: “Although there are now sophisticated sequencing techniques, it was in situ hybridization what guided us to unveil the mechanism”.
This work has been made possible thanks to the financial support provided by different institutions: the Spanish Ministry of Science, Innovation, and Universities; the Carlos III Health Institute; the ISIC and PROMETEO Programmes of the Generalitat Valenciana; the Spanish Ministry of Education, Culture and Sports; the Severo Ochoa Program for Centers of Excellence in Research and Development of the Spanish State Research Agency; the European Research Council; and the CERCA Program of the Generalitat of Catalunya.
Source: Institute for Neurosciences CSIC-UMH (in.comunicacion@umh.es)

 Español
Español

