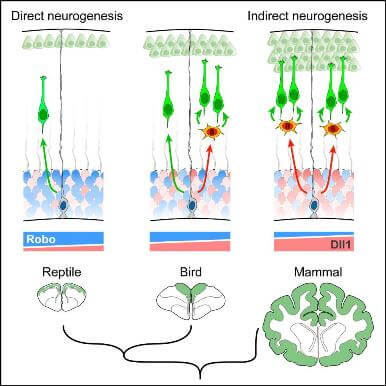
Evolution of Cortical Neurogenesis in Amniotes Controlled by Robo Signaling Levels
Cerebral cortex size differs dramatically between reptiles, birds and mammals owing to developmental differences in neuron production. In mammals, signaling pathways regulating neurogenesis have been identified, but genetic differences behind their evolution across amniotes remain unknown. We show that direct neurogenesis from Radial Glia Cells, with limited neuron production, dominates the avian, reptilian and mammalian paleocortex, whereas in the evolutionarily recent mammalian neocortex most neurogenesis is indirect via basal progenitors. Gain- and loss-of-function experiments in mouse, chick and snake embryos, and human cerebral organoids, demonstrate that high Slit/Robo – low Dll1 signaling, via Jag1/2, is necessary and sufficient to drive direct neurogenesis. Attenuating Robo signaling and enhancing Dll1 in snakes and birds recapitulates the formation of basal progenitors and promotes indirect neurogenesis. Our study identifies modulation in activity levels of conserved signaling pathways as a primary mechanism driving the expansion and increased complexity of the mammalian neocortex during amniote evolution.

 Español
Español