A right-handed signalling pathway drives heart looping in vertebrates
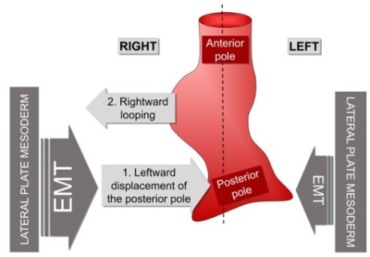
The heart is positioned on the left side due to left-right asymmetric cell movements directed to the midline of the embryo that displace the heart to the left.
Our bodies show an external bilateral symmetry. However, there are many internal asymmetries. As such, our liver is on the right while the spleen and heart are on the left. During embryonic development, all these organs appear at the midline but then they are displaced to their final position. This process is essential for correct organ packaging and function. The heart must have its posterior pole pointing to the left in order to achieve the proper concordance with the vasculature.
Defects in L/R asymmetry arise in 1/10,000 humans, and the associated morbidity and mortality usually imply congenital heart defects (CHDs).
This work has been carried out at the Instituto de Neurociencias (CSIC-UMH), led by Angela Nieto, and with the collaboration of researchers from the University of Malaga, the Molecular Biology Institute in Barcelona (IBMB-CSIC) and the University of Dresden.
Abstract
The majority of animals show external bilateral symmetry, precluding the observation of multiple internal left-right (L/R) asymmetries that are fundamental for organ packaging and function. In vertebrates, left identity is mediated by the left-specific Nodal-Pitx2 axis that is repressed on the right-hand side by the epithelial-mesenchymal transition (EMT) inducer Snail1. Despite some existing evidence, it remains unclear whether an equivalent instructive pathway provides right-hand specific information to the embryo. Here we show that in zebrafish, BMP mediates the L/R asymmetric activation of another EMT inducer, Prrx1a, in the lateral plate mesoderm (LPM) with higher levels on the right. Prrx1a drives L/R differential cell movements towards the midline leading to a leftward displacement of the cardiac posterior pole through an actomyosin-dependent mechanism. Downregulation of Prrx1a prevents heart looping and leads to mesocardia. Two parallel and mutually repressed pathways, respectively driven by Nodal and BMP on the left and right LPM, converge on the asymmetric activation of Pitx2 and Prrx1, two transcription factors that integrate left and right information to govern heart morphogenesis. This mechanism is conserved in the chicken embryo and, in the mouse, Snail1 fulfills the role played by Prrx1 in fish and chick. Thus, a differential L/R EMT produces asymmetric cell movements and forces, more prominent from the right, that drive heart laterality in vertebrates.

 Español
Español