A new dawn for gene therapy?
7 de July de 2023
Virus-based gene therapies: a powerful tool for preclinical research and an opportunity for human medicine.
Technically, gene therapy consists in the efficient delivery of a gene to a specific target tissue or cell type in a patient to treat a genetic disease. This genetic delivery can be carried out by viral vectors, including lentiviruses, adenoviruses or adeno-associated viruses (AAVs) injected in vivo to patients or alternatively, by the autologous transplant of genetically engineered cells, a technique employed in cancer immunotherapy with CAR(T) cells (reviewed [1]). Nowadays, viral-based gene therapies are routinely used in biomedical laboratories for preclinical research in animal models. However, its history in human clinical therapies has been marked by many transitions during the last 40 years.
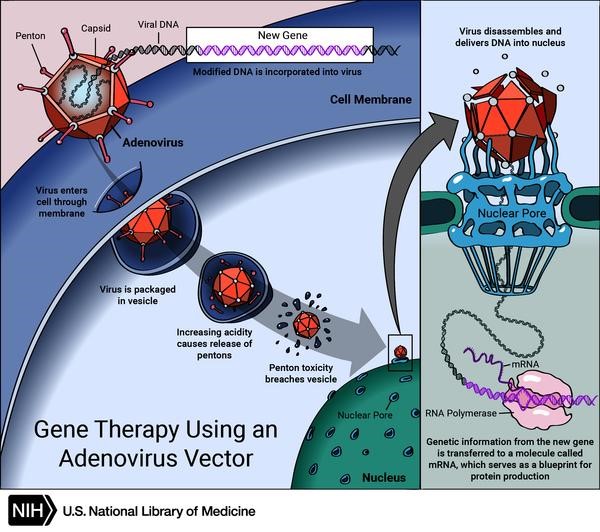
Scheme of gene therapy using an adenovirus vector. Credit: US National Library of Medicine
The first successful viral-based gene therapy in humans took place in the early 90s, when a patient suffering of adenosine deaminase (ADA) immunodeficiency was treated with infusions of T cells transformed by a recombinant retrovirus carrying the ADA gene [2]. Back then, this technology was meant to transform medicine and many viral-based gene therapies for treating human monogenic diseases were conducted. But these first-generation therapies were strongly immunogenic for human patients, which restricted the delivery and expression of exogenous genes and also caused undesired immune responses in treated subjects. Indeed, the death in 1999 of a volunteer patient enrolled in a clinical trial for ornithine transcarbamylase (OTC) deficiency gene therapy with adenovirus, due to a cytokine storm reaction, raised safety concerns and halted funding and most research in the field [3]. In more recent years, nevertheless, new advances in our understanding of viral biology have reignited interest in virus-based gene therapies, mainly due to the identification of “rare” human serotypes with low seroprevalence or the characterization of the adeno-associated viruses (AAVs). AAVs present low immunogenicity in humans and display a broad spectrum of tissue-specific serotypes, which together with capsid engineering and gene promoter design, enable the specific transduction of multiple tissue/cell types.
To date, three AAV-based gene therapy drugs worldwide have made it to the commercial market: Glybera (Amsterdam Molecular Therapeutics), an AAV1-based gene therapy that delivered the lipoprotein lipase (LPL) gene to patients with LPL deficiency; Luxturna (Spark Therapeutics), an AAV2-based vector that delivers the retinoid isomerohydrolase RPE65 gene to prevent progressive blindness in Leber’s congenital amaurosis; Zolgensma (Novartis), a one-time gene therapy that delivers SMN1 gene to patients with spinal muscular atrophy. Moreover, more than hundred clinical trials based on AAV are being conducted worldwide aiming from blood disorders to eye conditions [4]. For example, the French companies SparingVision and Vivet Therapeutics are developing AAV-based treatments aiming to inherited retinal diseases and orphan metabolic diseases, respectively; and the Spanish spin-off Splice Bio, studies novel viral-based gene therapies based on its proprietary intein platform technology to treat patients suffering from incurable genetic diseases. Indeed, a recent case of success in the Spanish biotech ecosystem has been Viralgen (Gipuzkoa Science and Technology Park), one of the world's leading manufacturers of cGMP-certified AAVs for gene therapy treatments of human patients. Viralgen has been recently acquired by Bayer in the largest financial transaction in Spanish biotech history (EUR 4,000 M), mediated by the Valencian venture capital firm Columbus Venture Partners.
At the Instituto de Neurociencias (CSIC-UMH), the Neurotropic Vectors Unit presents a great opportunity to develop new research and ideas for virus-based gene therapy in preclinical research. The Neurotropic Vectors Unit offers a service focused on the production of customized viral vectors for researchers, which are highly valuable tools to manipulate genetic expression in brain cells.
References
[1] J. T. Bulcha, Y. Wang, H. Ma, P. W. L. Tai, and G. Gao, “Viral vector platforms within the gene therapy landscape,” Signal Transduct Target Ther, vol. 6, no. 1, p. 53, Feb. 2021, doi: 10.1038/s41392-021-00487-6.
[2] W. F. Anderson, “Human gene therapy,” Science, vol. 256, no. 5058, pp. 808–813, May 1992, doi: 10.1126/science.1589762.
[3] S. E. Raper et al., “Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer,” Mol Genet Metab, vol. 80, no. 1–2, pp. 148–158, 2003, doi: 10.1016/j.ymgme.2003.08.016.
[4] G. Conroy, “How gene therapy is emerging from its ‘dark age,’” Nature, vol. 612, no. 7940, pp. S24–S26, Dec. 2022, doi: 10.1038/d41586-022-04210-5.

 Español
Español