Researchers discover how the interaction of two “epigenetic guardians” protects neuronal identity
10 de September de 2025
Researchers discover how the interaction of two “epigenetic guardians” protects neuronal identity
- This study shows that the enzymes KDM1A and KDM5C collaborate to prevent neurons from activating inappropriate genes, ensuring their proper function.
- The findings, published in Cell Reports, open new avenues to understand the origin of neurological disorders associated with mutations in these genes, such as intellectual disability.

Photo: Sergio Niñerola, Ángel Barco, Juan Paraíso Luna, and Beatriz del Blanco, IN CSIC-UMH researchers
Neurons are highly specialized cells, and their proper functioning depends on preserving their identity throughout life. The team from the Transcriptional and Epigenetic Mechanisms of Neuronal Plasticity laboratory, led by Ángel Barco at the Institute for Neurosciences (IN), a joint center of the Spanish National Research Council (CSIC) and the Miguel Hernández University (UMH) of Elche, has identified that two enzymes, KDM1A and KDM5C, interact to act as true “epigenetic guardians”. Their role is to silence genes that do not belong to neurons and to keep only the appropriate instructions active.
To carry out this study, recently published in Cell Reports, the team used a mouse model in which they simultaneously deleted the KDM1A and KDM5C genes in neurons of the adult brain. This allowed them to investigate what happens when this epigenetic control is lost in mature neurons, and not only during development. “What is surprising is that the joint action of these two enzymes goes beyond the sum of their individual effects”, says Barco, explaining: “When both fail, the neurons begin to express genes that do not belong to it, with negative consequences for memory, learning ability, and the animal’s regulation of anxiety”.
Through a multidisciplinary approach combining genetics, molecular biology, electrophysiology, super-resolution microscopy, behavioral studies, and large-scale genomic analyses, the researchers observed that the loss of both enzymes profoundly alters the neuron’s epigenetic landscape: several genomic regions accumulated an epigenetic mark (H3K4me3), usually associated with active genes, in areas that should remain inactive. They also detected a disorganization in the three-dimensional structure of the neuronal genome. These changes result in alterations in neuronal physiology, such as increased excitability, which negatively impact the behavior and cognitive abilities of the mice.
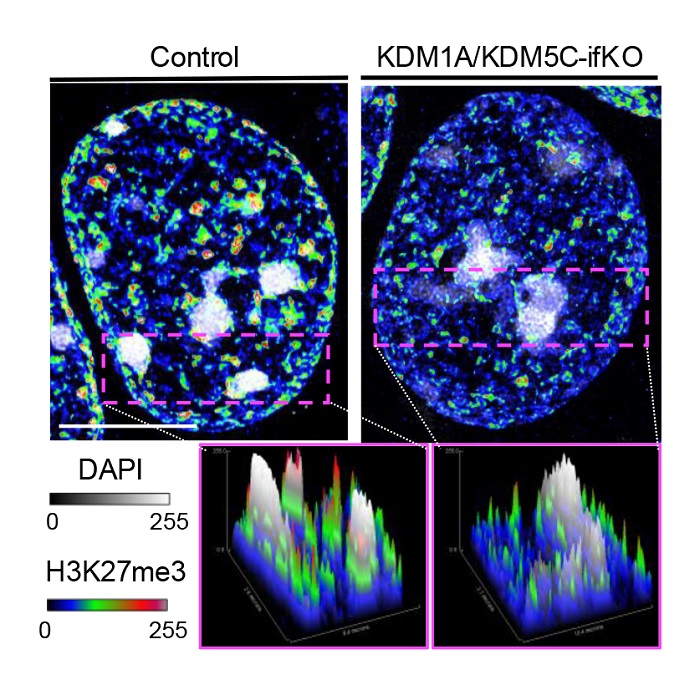
Images of the nucleus of an excitatory neuron of a control mouse (left) and a mouse in which KDM1A and KDM5C have been simultaneously deleted in the adult brain. The DAPI signal (DNA) is shown in gray, and the H3K27me3 signal, an epigenetic mark associated with repression, is shown in color. Source: Cell Reports.
These results represent progress in understanding the origin of neurological disorders associated with intellectual disability caused by mutations in epigenetic regulators: “Understanding how these enzymes interact not only helps us decipher the biology of neurons, but also to identify possible mechanisms involved in neurological diseases”, highlights Juan Paraíso Luna, co-first author of the article.
This study complements previous work from the same laboratory that had already demonstrated the relevance of each of these enzymes individually: KDM1A, essential to preserve the three-dimensional organization of the genome and prevent its deterioration associated with aging, and KDM5C, necessary to avoid erroneous transcriptions and to fine-tune neuronal responses to stimuli. The novelty now is that both proteins work together to maintain neuronal identity. “Mutations in the Kdm1a and Kdm5c genes have been associated in humans with intellectual disability and other neurological disorders, so this work opens the door to new research that can help us delve deeper into the origin of certain brain diseases”, concludes Barco.
"Cooperative control of neuron-specific repressive chromatin states by intellectual-disability-linked KDM1A and KDM5C demethylases." Martín-González AM, Paraíso-Luna J, Niñerola S, Del Blanco B, Robles RM, Herrera ML, Muñoz-Viana R, Geijo-Barrientos E and Barco A. Cell Reports. 2025 44, 116201. DOI https://doi.org/10.1016/j.celrep.2025.116201
This work was made possible thanks to collaboration with the group of Professor Emilio Geijo, from the UMH Department of Physiology, and funding from La Marató de TV3, the Spanish State Research Agency, the Generalitat Valenciana, and the “LaCaixa” Foundation.
Source: Institute for Neurosciences CSIC-UMH (in.comunicacion@umh.es)

 Español
Español