Possible Mechanism for Fold Formation in the Cerebral Cortex Discovered
6 de June de 2024
– This work shows that epigenetic marks are a key mechanism in the instructions that genes receive to express themselves and generate folds.
– The Cux2 protein can alter the pattern of brain folding in ferrets and induce it in mice.
"Gene regulatory landscape of cerebral cortex folding2". Singh A, Del-Valle-Anton L, de Juan Romero C, Zhang Z, Fernández Ortuño E, Mahesh A, Espinós A, Soler R, Cárdenas A, Fernández V, Lusby R, Tiwari V K and Borrell V. Science Advances, 2024, 10(23), eadn1640.
DOI: https://doi.org/10.1126/sciadv.adn1640

(Photo: Lucía del Valle Antón, Eduardo Fernández Ortuño, Víctor Borrell, Virginia Fernández, Rafael Soler y Adrián Cárdenas, IN-CSIC-UMH researchers)
Determining the genetic and epigenetic factors that influence brain folding is the objective of the latest study co-led by the Neurogenesis and Cortical Expansion laboratory, directed by researcher Víctor Borrell at the Institute of Neurosciences (IN), a joint center of the Spanish National Research Council and the Miguel Hernández University (UMH) of Elche, and the laboratory led by researcher Vijay K. Tiwari at the Wellcome-Wolfson Institute of Experimental Medicine at Queen’s University of Belfast (UK). This work, published in the journal Science Advances, has shown that epigenetic marks are a key mechanism in the instructions that give rise to the folds of the cerebral cortex and that the Cux2 protein plays a determining role in this process.
Borrell’s team had already developed a protomap that establishes at the genetic level where the gyri and sulcus will be generated in the brain during a stage of embryonic development in which the folds have not yet begun to be generated. “At first, the cortex is smooth, but there is an area that will grow a lot, and as it grows, it will generate a gyrus. Meanwhile, next to it, other areas will grow less and will remain sunken, forming a sulcus”, explains the researcher and adds: “This is because there are thousands of genes that are expressed in the cortex of the embryo while it is developing. Still, they are not expressed in the same amount in all areas”.
Thanks to the collaboration with Tiwari’s laboratory, an expert in epigenetics and epigenomics analysis, they have been able to take this research one step further and study what is known as the epigenetic landscape of the cells of the cerebral cortex: “We have studied much more than a specific gene in a particular location, but we have been able to observe all DNA from cells and their epigenetic modifications, which determine the behavior of these genes, in order to understand the mechanisms that give rise to the expression of those genes” highlights Borrell.
To develop this study, the researchers focused on the epigenetic mark H3K27ac, as it is the indicator with the greatest ability to predict gene expression. However, the results were surprising: “We observed that in many places where H3K27ac was present, gene expression did not occur and we also observed the opposite case, there were genes that were expressed without the epigenetic mark being present,” says Lucía del Valle Antón, co-first author of the article. Experts agree that this finding is a clear indicator of the complexity of the nervous system: “In the field of epigenetics, we find evidence that suggests that the nervous system during its development is an exception and does not function in the same way as the rest of the body tissues. Without a doubt, there is a long way ahead to study and it is an exciting challenge,” highlights Borrell.
This unexpected finding led them to investigate what was happening in those genes in which there was a coincidence between the H3K27ac mark and the expression. To do this, they focused on proteins that directly regulate how much genes are expressed: the transcription factors. Specifically Cux2 protein, because its participation in brain development is widely known.
Cux2, a master factor
Cux2 is a protein involved in neuronal differentiation, dendrite growth, and the formation of neuronal circuits in general. The experts wanted to verify the influence of this factor on brain folding and to do so they introduced the DNA that encodes this protein into the brain of the embryo during its gestation. Thanks to this technique, they confirmed that Cux2 is capable of altering folding patterns: “It can generate folds in the mouse cerebral cortex, which is otherwise smooth, and in the case of the ferret, which already has folds, the protein can completely change the established folding pattern”, explains del Valle Antón.
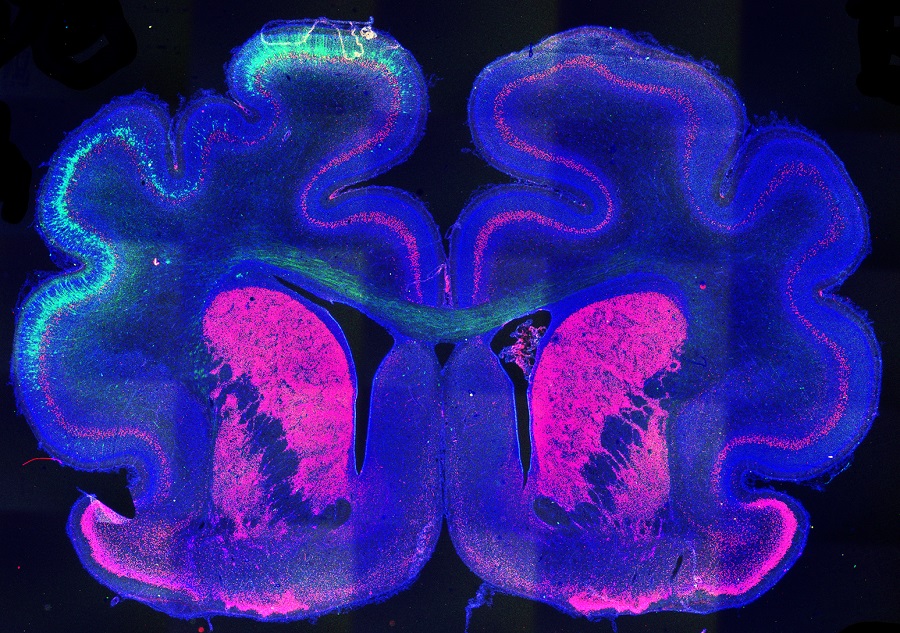
Image of a ferret brain showing the altered folding pattern (left) mediated by the Cux2 protein.
These results reveal the key role of Cux2 in folding: “We know that for folds to form, multiple processes have to occur and, after carrying out this study, we have determined that Cux2 is a master factor that can take advantage of the epigenetic landscape to make the changes that lead to the expression of thousands of genes that carry out different tasks. The combination of all this makes possible the formation of folds”, explains Borrell.
Through single-cell sequencing, the researchers could analyze the changes that Cux2 causes in cells to generate the gyri. They verified that there is a type of radial glia cell, the stem cells responsible for generating neurons, that practically disappears, allowing other types of radial glia cells to proliferate in greater quantities. This not only affects the type of progenitor that gives rise to neurons but also the cell lineage they follow, which in turn is directly involved in the development of gyri and sulci in the brain.
Folding is a characteristic of the human brain that, when defective, leads to serious learning and intellectual disabilities. Sometimes, patients have genetic mutations that cause brain malformations due to the lack of gyri. In this regard, Borrell points out that conducting basic research “is essential to understanding the biology behind these diseases and allows us to be a little closer to finding possible solutions”.
This work has been possible thanks to funding from the European Research Council (ERC) within the framework of the Horizon Europe program of the European Union, the Spanish State Research Agency – Spanish Ministry of Science, Innovation and Universities through the project programs of Generación de conocimiento, FPI, and Juan de la Cierva, the “Severo Ochoa” Programme for Centers of Excellence in R&D, the “La Caixa” Foundation, the German Research Society (Deutsche Forschungsgemeinschaft), the Novo Nordisk Foundation and the Danish National Research Foundation (DNRF).
This research is part of the UNFOLD project ‘Unfolding the dynamic interaction between mechanical and molecular processes in brain folding’ (ERC-2023-SyG n°101118729), whose objective is to study cortical folding from the point of view of mechanics, cell biology, and genetics.
Source: Institute for Neurosciences CSIC-UMH (in.comunicacion@umh.es)

 Español
Español