Global and local nature of cortical slow waves.
A study by the Institute for Neurosciences CSIC-UMH reveals how the brain organizes and directs its slowest activity
• The study, published in iScience, shows that the directionality of slow waves in the cerebral cortex depends on neuronal excitability and not only on anatomy.
• The results of this work, led by the Institute for Neurosciences CSIC-UMH, could help to better understand states such as deep sleep, anesthesia, or pathologies like epilepsy.
(Photo: The researchers from the Institute for Neurosciences CSIC-UMH Ramón Reig, Javier Alegre Cortés, and María Sáez.)
The brain never rests: even during deep sleep or under anesthesia, it maintains rhythmic electrical activity known as slow oscillations. A team from the Sensory-motor Processing by Subcortical Areas laboratory, led by Ramón Reig at the Institute for Neurosciences, a joint center of the Spanish National Research Council (CSIC) and the Miguel Hernández University (UMH) of Elche, has discovered what determines the direction of these waves. The study, published in iScience, reveals that the key lies not in anatomical structure, as previously thought, but in the degree of neuronal excitability.
The discovery was made possible thanks to an advanced computational model that combines two levels of analysis: the local activity of isolated neural networks and the global interaction between different brain areas. “Until now, most studies have worked on those two scales separately. The novelty of our approach is that we analyzed them together, which allowed us to see how local differences fade when networks are connected”, explains Reig, who co-led the study with researcher Javier Alegre Cortés.
The model showed that when several brain areas connect, differences among them tend to synchronize, following the rhythm set by the most excitable region. “It’s like what happens in a classroom: each student may have their own style, but if someone sets a trend, the others end up following”, Alegre illustrates. This concept of a neuronal ‘leader’ helps explain why, despite the diversity of properties across brain regions, slow waves ultimately propagate in a coordinated manner.
The researchers demonstrated that brain slow waves are not guided solely by anatomy, but also by the degree of excitability of specific neurons. “Our model predicted that the direction of oscillations depended on which neuronal group was most excitable at a given moment, and we confirmed it with experiments in mice”, notes Reig. When they increased excitability in the occipital lobe of anesthetized mice by applying a cocktail of drugs that made neurons more active, they observed that the wave direction reversed: instead of traveling from the front to the back of the brain, they moved the other way around.
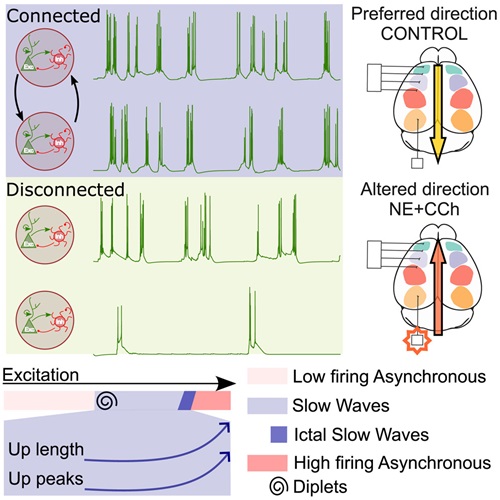
Graphical abstract of the study. Source: iScience
Under normal conditions, these oscillations play an essential role during deep sleep and anesthesia, as they help organize brain activity while at rest. However, when the mechanisms regulating them are altered, they may appear during wakefulness or transform into electrical patterns associated with epilepsy: “Understanding how excitability modulates these waves also provides clues to what happens when neuronal activity gets out of control”, the authors point out. In this study, simulations were carried out modifying the main factors that impact slow-wave activity in isolated or interconnected regions. The simulations successfully replicated different brain activity states, describing which factors are relevant at the local level and which at the global level.
Beyond the findings, this work also represents a methodological advance. The model used by the team is based on real data about the anatomy and physiology of the mammalian brain, enabling realistic simulations of how neural networks behave when connected. “Mathematical models complement experiments, making it possible to explore scenarios that are difficult to reproduce in the lab and to rigorously test hypotheses”, Alegre highlights.
This work involved the collaboration of Maurizio Mattia from the National Center for Radiological Protection and Computational Physics in Rome (Italy) and was made possible thanks to funding from the Spanish State Research Agency, through the Severo Ochoa Centers of Excellence Program; the Ministry of Science, Innovation and Universities; the Miguel Hernández University through the Margarita Salas fellowship program; the Generalitat Valenciana; and Italy’s National Recovery and Resilience Plan (PNRR), funded by the European Union (NextGenerationEU).
Source: Institute for Neurosciences CSIC-UMH (in.comunicacion@umh.es)


 Español
Español