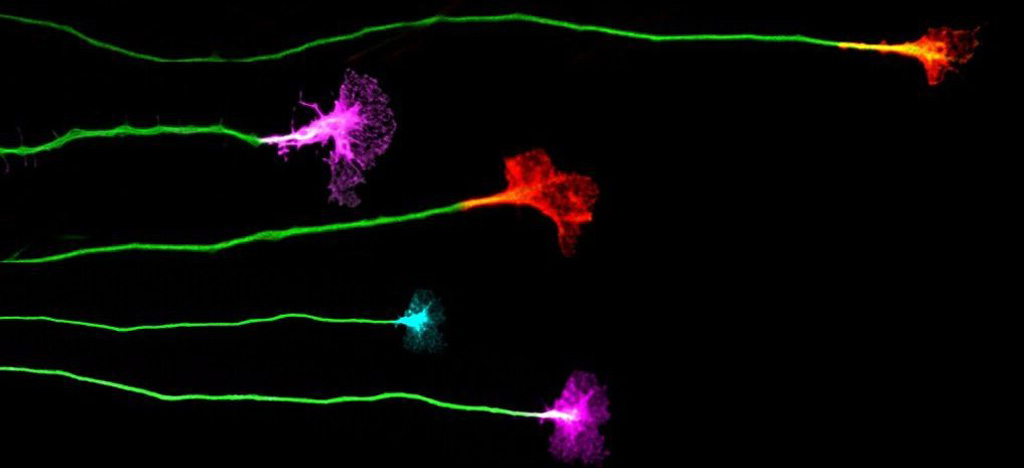
EphA4 mediates ephrinB1-dependent adhesion in retinal ganglion cells.
A study by the Institute for Neurosciences CSIC-UMH reveals new mechanisms essential for the visual circuit development
• The EphA4 protein, a key player in the organization of the visual system, performs unexpected functions as a ‘cellular glue’ during embryonic development.
• The findings of this study, published in the Journal of Neuroscience, contribute to a better understanding of axonal adhesion’s critical role in forming the visual circuit.
(Photo: Researchers Verónica Murcia Belmonte and Eloísa Herrera. Source: IN CSIC-UMH)
A team of researchers from the Institute for Neurosciences (IN), a joint center of the Spanish National Research Council (CSIC) and the Miguel Hernández University (UMH) in Elche, has identified a novel role for the EphA4 protein in the development of the visual system. This discovery challenges established theories on axonal guidance by demonstrating that, besides repelling axons, the EphA4 receptor can promote adhesion when it binds to ephrinB1. This mechanism, which functions as a ‘cellular glue’ during development, is essential for retinal ganglion cell axons to connect with specific structures in the brain, organizing the visual map accurately.
The study, led by researcher Eloísa Herrera, head of the Generation and Regeneration of Bilateral Neural Circuits laboratory at the IN, was recently published in The Journal of Neuroscience. The findings of this research could have implications beyond vision, contributing to the understanding of cellular migration mechanisms and the development of other cellular processes in embryonic development.
A Paradigm Shift in Axonal Guidance
Retinal ganglion cells are neurons that transmit visual information from the eye to the brain. During embryonic development, these cells extend long axons that must reach specific regions in the brain, guided by signaling molecules. Eph proteins and their ligands, ephrins, function as a sophisticated molecular positioning system that enables neurons to navigate during development. Ephrins act as molecular GPS, indicating the directions neuronal axons should follow, while Eph proteins interpret these signals to guide their movement.
Until now, interactions between Eph proteins and ephrins were believed to mediate only axonal repulsion responses, directing their trajectories. However, this new study reveals that EphA4, in combination with one of its ligands, ephrinB1, generates an adhesion response critical for axonal anchoring. “We have discovered that, under certain conditions, EphA4 changes its function, shifting from repelling axons to promoting their adhesion,” explains Eloísa Herrera.
Mouse retina showing ganglion cells that overexpress EphA4 forming aggregates (green) and their axons labeled in red, which are crucial for the proper connectivity of the visual system. Source: The Journal of Neuroscience.
Promoting adhesion is crucial for axons to anchor in the correct location, ensuring an accurate visual map. In this context, researcher Verónica Murcia, the article’s first author, highlights: “The cellular mechanisms controlling the formation of neural circuits are far more versatile than we imagined. It is fascinating that the same protein can act as a traffic light, sometimes stopping and other times facilitating passage, depending on the ligand it binds to”.
To conduct this study, the team combined advanced genetic and imaging techniques with the use of a genetically modified mouse model in which the EphA4 protein expression was eliminated. Additionally, through in-utero electroporation, they were able to label and track individual axons from the retina to the superior colliculus, a brain nucleus responsible for organizing the visual map. “This technique allowed us to precisely analyze how the absence of EphA4 specifically affects certain axons in particular regions of the developing brain”, adds the researcher.
The results showed that, without EphA4, axons from specific regions of the retina failed to properly connect to the corresponding brain areas where they must form a map. “These experiments not only confirm the importance of EphA4 in axonal adhesion but also suggest that this mechanism could be relevant to other embryonic developmental processes where this protein is also prominently expressed, such as the formation of somites, which are transient structures formed on either side of the neural tube during embryonic development that give rise to the cells that will form the vertebrae and ribs, the dermis of the dorsal skin, and the skeletal muscles of the back and limbs”, Herrera points out.
This work has been made possible thanks to the financial support provided by the Spanish State Research Agency (AEI) – Ministry of Science, Innovation, and Universities, the PROMETEO program of the Generalitat Valenciana, the ”la Caixa” Foundation, the project CelMa-ENVEJECE by ICAR Foundation, and the Severo Ochoa Program for Centers of Excellence.
Source: Institute for Neurosciences CSIC-UMH (in.comunicacion@umh.es)


 Español
Español