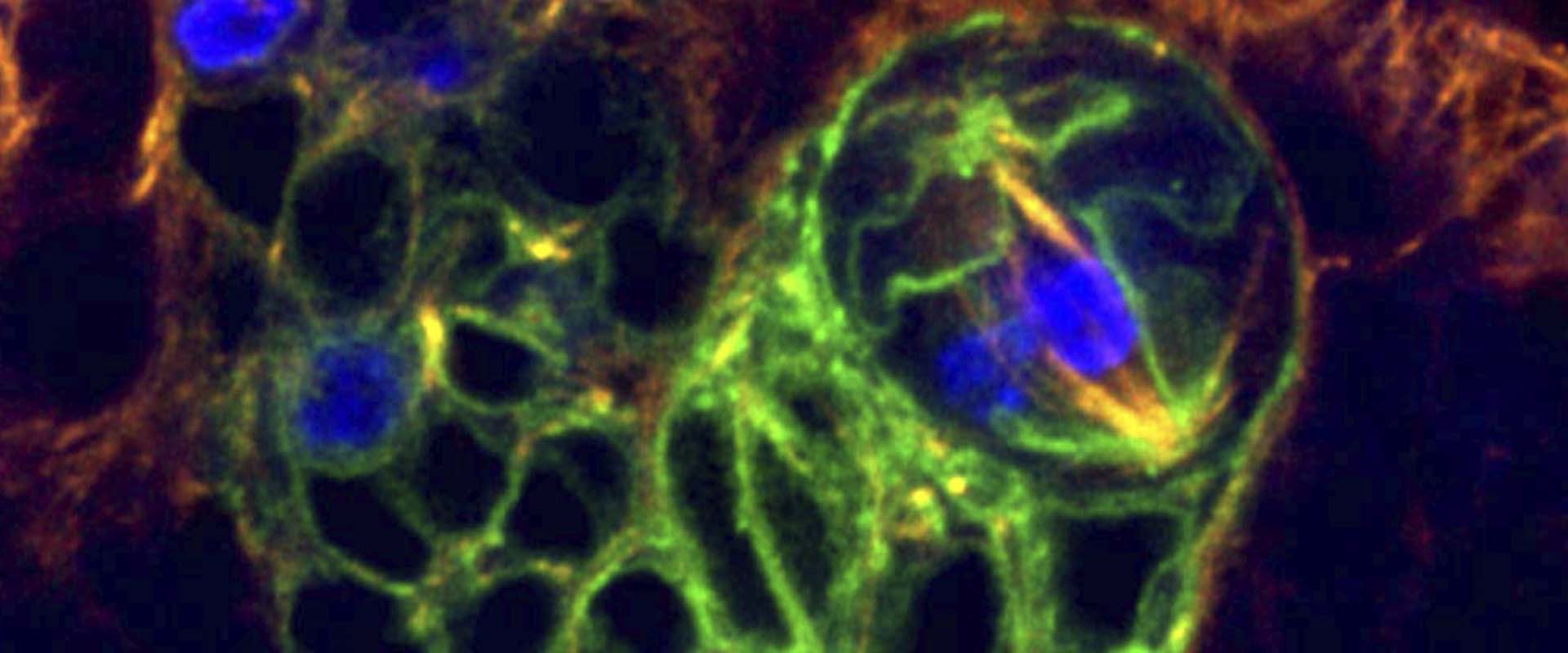
Eph signaling controls mitotic spindle orientation and cell proliferation in neuroepithelial cells
Mitotic spindle orientation must be tightly regulated during development and adult tissue homeostasis. It determines cell-fate specification and tissue architecture during asymmetric and symmetric cell division, respectively. Here we uncover a novel role for Ephrin-Eph intercellular signaling in controlling mitotic spindle alignment in Drosophila optic lobe neuroepithelial cells, through aPKC activity-dependent myosin II regulation. We show that conserved core components of the mitotic spindle orientation machinery, including Discs Large1, Mud/NuMA and Canoe/Afadin, mislocalize in dividing Eph mutant neuroepithelial cells and produce spindle alignment defects in these cells when they are downregulated. In addition, the loss of Eph leads to a Rho signaling-dependent activation of the PI3K/Akt1 pathway, enhancing cell proliferation within this neuroepithelium. Hence, Eph signaling is a novel extrinsic mechanism that regulates both spindle orientation and cell proliferation in the Drosophila optic lobe neuroepithelium. Similar mechanisms could operate in other Drosophila and vertebrate epithelia.

 Español
Español