Class IIa histone deacetylases link cAMP signaling to the myelin transcriptional program of Schwann cells
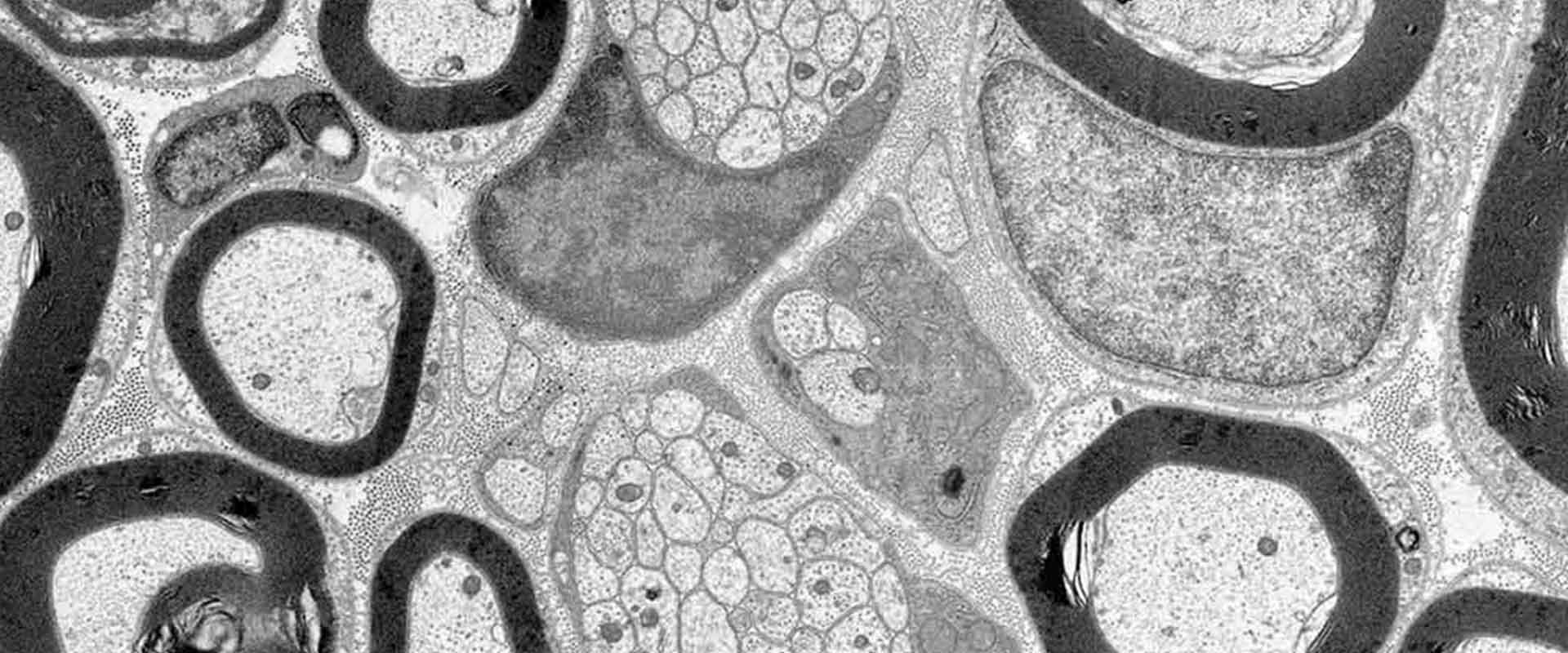
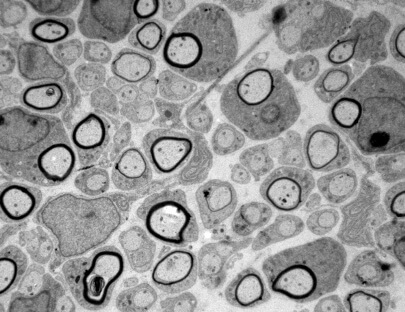
Schwann cells respond to cyclic adenosine monophosphate (cAMP) halting proliferation and expressing myelin proteins. In this work authors show that cAMP signaling induces the nuclear shuttling of the class IIa histone deacetylase (HDAC)–4 in these cells, where it binds to the promoter and blocks the expression of c-Jun, a negative regulator of myelination. To do it, HDAC4 does not interfere with the transcriptional activity of MEF2. Instead, by interacting with NCoR1, it recruits HDAC3 and deacetylates histone 3 in the promoter of c-Jun, blocking gene expression. Importantly, this is enough to up-regulate Krox20 and start Schwann cell differentiation program, inducing myelin gene expression. Using conditional knockout mice, authors also show that HDAC4 together with HDAC5 redundantly contribute to activate the myelin transcriptional program and the development of myelin sheath in vivo. A model is proposed in which cAMP signaling shuttles class IIa HDACs into the nucleus of Schwann cells to regulate the initial steps of myelination in the peripheral nervous system.

 Español
Español