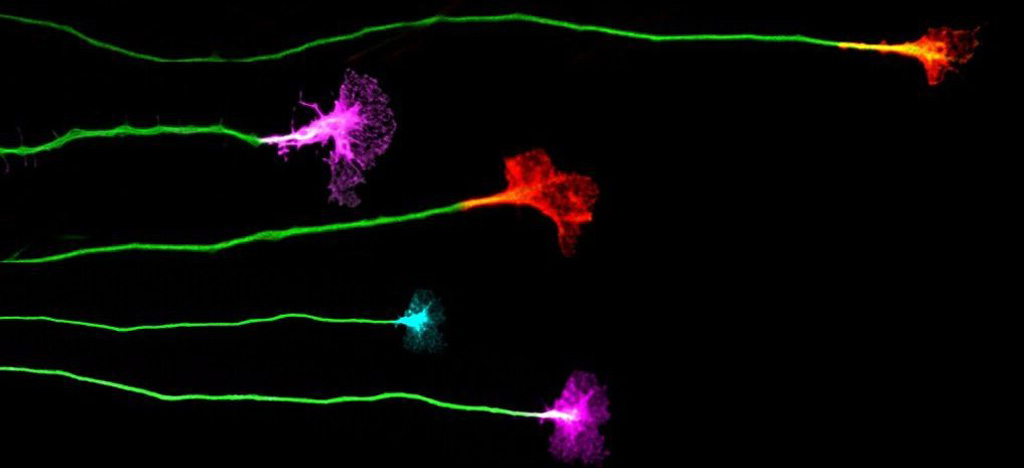
ARID1A-BAF coordinates ZIC2 genomic occupancy for epithelial-to-mesenchymal transition in cranial neural crest specification.
A study co-led by the Institute for Neurosciences CSIC-UMH uncovers a key link in the genetic communication of craniofacial development
• Experts have discovered that the ARID1A gene regulates an essential genetic program for cell migration, with ZIC2 identified as one of the most crucial genes in this process.
• These findings provide new insights into the craniofacial defects characteristic of Coffin-Siris Syndrome and could pave the way for addressing other developmental disorders.
(Photo: Eloísa Herrera, CSIC Research Professor at the Institute for Neurosciences CSIC- UMH)
An international team of researchers has identified a key genetic mechanism that regulates the formation and migration of cranial neural crest cells, which are essential for developing facial structures. This discovery, published in The American Journal of Human Genetics, expands our understanding of the roles played by specific genes in a critical step of embryonic development and paves the way for deeper insights into the genetic causes of certain congenital diseases.
This study, co-led by Eloísa Herrera, head of the Generation and Regeneration of Bilateral Neural Circuits laboratory at the Institute of Neurosciences (IN), a joint center of the Spanish National Research Council (CSIC) and the Miguel Hernández University (UMH) of Elche, and Marco Trizzino, whose laboratory at Imperial College London specializes in the study of human stem cells, has revealed how the ZIC2 gene, in collaboration with the ARID1A-BAF complex, plays a crucial role in a process known as epithelial-to-mesenchymal transition (EMT). This process enables cells to change shape and migrate to their destinations within the embryo to form organs and tissues, including facial structures.
International collaboration
The team conducted experiments using stem cells derived from patients with Coffin-Siris Syndrome (CSS), a rare genetic disorder caused by insufficient gene function. CSS is characterized by abnormalities in various parts of the body, including limb defects, intellectual disability, and craniofacial malformations. These cells were used to study how genetic alterations in ARID1A impact the genetic programs of EMT and the function of the ZIC2 gene. The analyses employed advanced techniques such as RNA-seq and ChIP-seq, which enabled the identification of the genes regulated by this molecular axis.
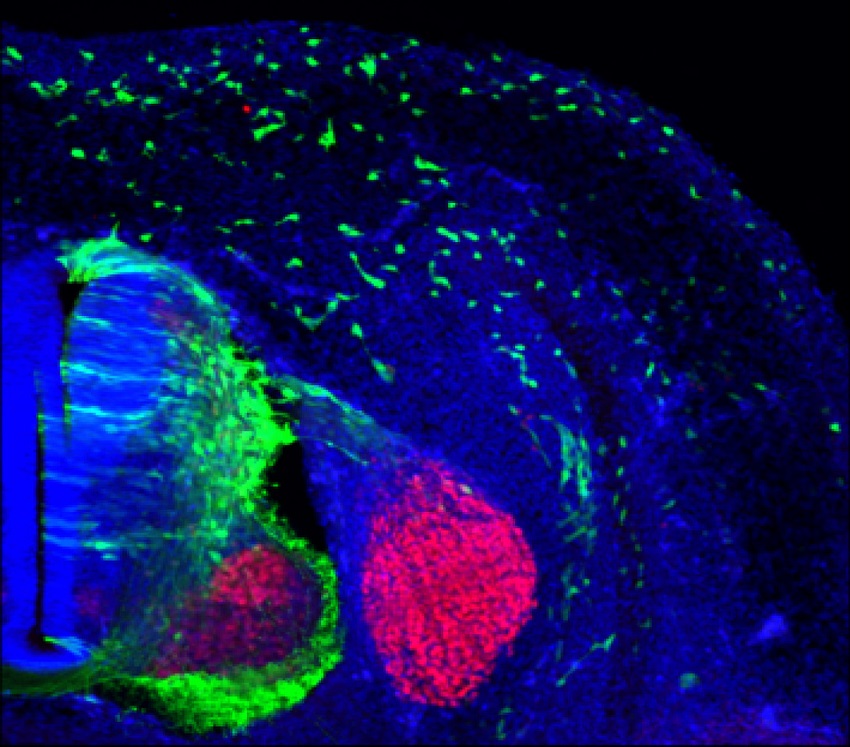
Photo: The ZIC2 gene drives the migration of neural crest cells (in green) during early embryonic stages, a process essential for forming the vertebrate nervous system. IN CSIC-UMH.
Additionally, the team used animal models, including mice and chicken embryos, to observe in vivo how ZIC2 regulates the migration of neural crest cells and to examine the defects associated with the loss of ARID1A during craniofacial development. “This is how we discovered that ZIC2 is expressed in premigratory neural crest cells, just before they begin migration”, notes Herrera.
The findings of this study reveal that ARID1A regulates an essential genetic program for EMT, with ZIC2 identified as one of the most critical genes in this process. If ARID1A is not functioning properly, ZIC2 cannot occupy the genomic places required to activate EMT-related genes, disrupting neural crest migration, triggering aberrant cellular trajectories, and leading to craniofacial defects.
This research sheds light on the genetic mechanisms underlying craniofacial development and provides valuable insights for developing targeted therapies. “Understanding how ZIC2 and ARID1A interact during development gives us a key tool to explore potential treatments for congenital genetic diseases”, Herrera concludes.
This work has been made possible thanks to the financial support provided by the Spanish State Research Agency (AEI) – Ministry of Science, Innovation, and Universities, the ”la Caixa” Foundation, the PROMETEO program of the Generalitat Valenciana, the Severo Ochoa Program for Centers of Excellence, The G. Harold and Leila Y. Mathers Foundation, and the National Institutes of Health (NIH) of the United States Department of Health and Human Services.
Source: Institute for Neurosciences CSIC-UMH (in.comunicacion@umh.es)


 Español
Español