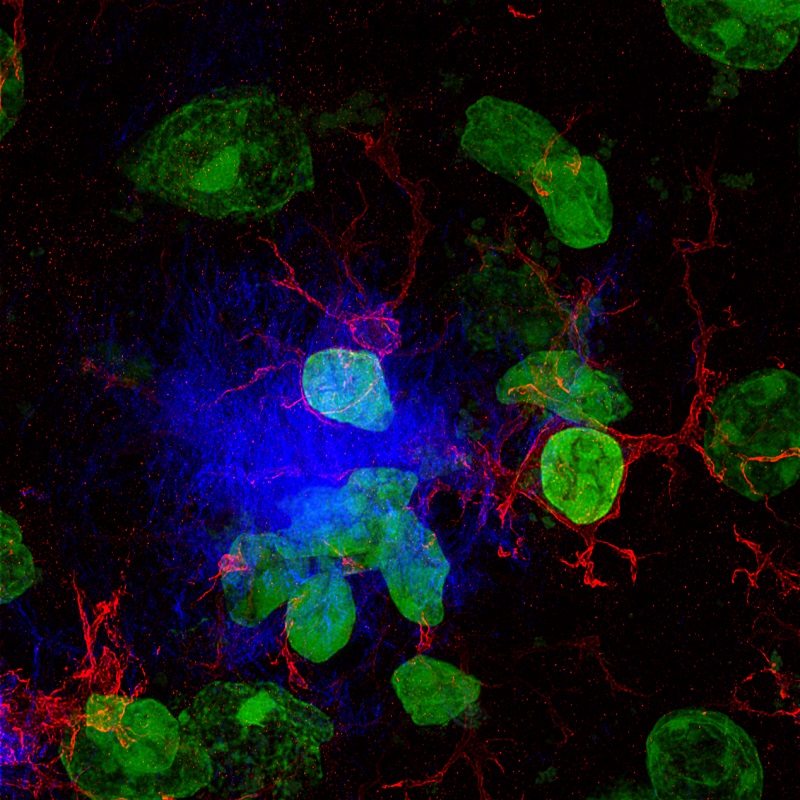
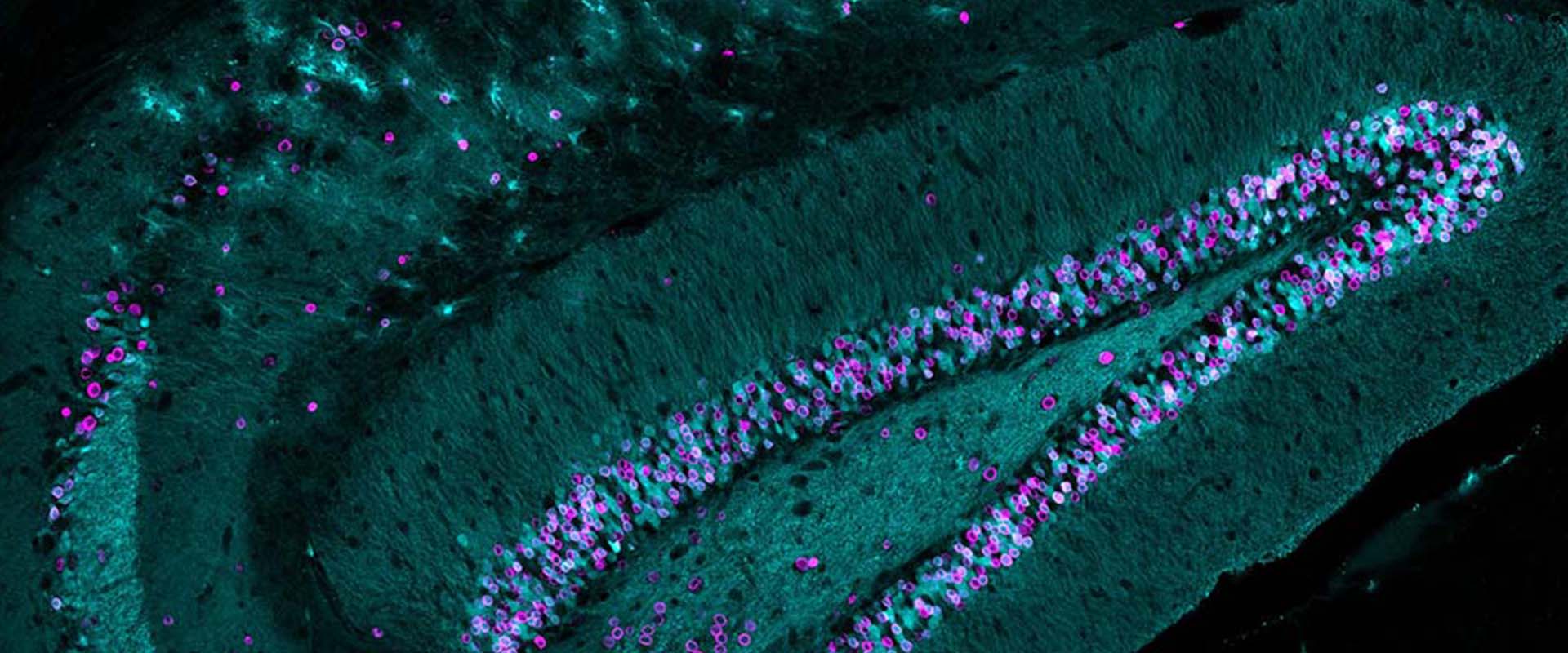
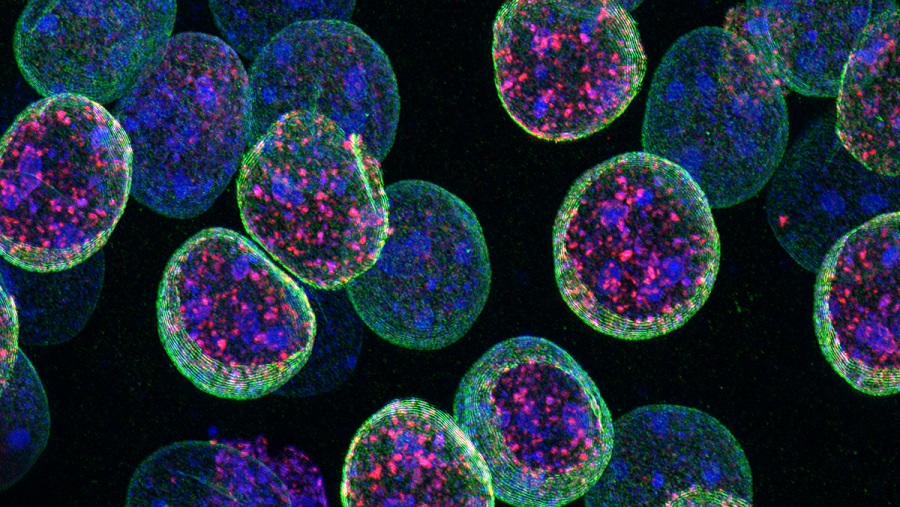
Kdm1a safeguards the topological boundaries of PRC2-repressed genes and prevents aging-related euchromatinization in neurons.
A study from the Institute for Neurosciences CSIC-UMH reveals the role of the protein Kdm1a in maintaining neuronal identity
• The identity of a cell determines its morphology and function throughout its lifespan. Studying the mechanisms that maintain neuronal identity is crucial to understanding rare neurological disorders and neurodegenerative diseases.
• This work, published in Nature Communications, suggests that natural aging in both mice and humans produces similar defects as the absence of the protein Kdm1a.
(Photo: Carina Racovac Farinha, Beatriz del Blanco Pablos, Angel Barco Guerrero, Juan Paraíso Luna y Sergio Niñerola Rives. IN-CSIC-UMH)
Epigenetic processes allow different cell types to emerge from a single genome. Throughout development, cells differentiate and acquire distinct characteristics by expressing the same genome in different ways. However, a less-known aspect of this process is how cells maintain their unique identities over time. A study led by the Transcriptional and epigenetic mechanisms of neuronal plasticity laboratory, headed by Angel Barco at the Institute for Neurosciences, a joint center of the Spanish National Research Council (CSIC) and the Miguel Hernández University (UMH) of Elche, has determined that the protein Kdm1a plays a crucial role in preserving the identity of neurons
Neurons are cells that have a very particular nuclear structure because once they are formed during development they never divide again, which is why neurons need to precisely maintain their identity throughout their lifespan. “Identity is given by what is done and what is not done; at an epigenetic level this translates into the genes that are expressed, but also in those that are not expressed” explains Angel Barco.
The results of this study, published in the journal Nature Communications, demonstrate that deleting Kdm1a in forebrain neurons in adult mice triggers the expression of genes that normally should not be expressed in neurons, which compromises neuronal identity. The researchers verified that in normal elderly mice, there is an incipient activation of the same genes derepressed in mice that have lost Kdm1a, indicating that natural aging reproduces the same defects as the lack of Kdm1a, although on a smaller scale. They found that eliminating this protein accelerates neuronal aging at an epigenetic level and alters gene transcription.
The experts correlated these findings with human data by collaborating with researcher José Vicente Sánchez Mut, who leads the Functional Epi-Genomics of Aging and Alzheimer’s Disease laboratory at the IN. They used a database of people between 50 and 80 years old and found that there is also a significant increase in the expression of some of these typically silent genes as people age.
Neurons from the brain of an adult mouse in which chromatin (in blue), the nuclear envelope (in green), and silenced regions of chromatin (in red) are labelled. IN-CSIC-UMH
Furthermore, this work shows that the repressive function of Kdm1a maintains the separation between genes that should be expressed and those that should be silent through the modulation of chromatin structure. The separation into “compartments” allows the maintenance of order throughout the lifespan of the neuron, which is essential to preserve its identity: “We know that disorder can have detrimental effects, both in aging and in intellectual disability because it blurs the barrier between what should be expressed and what should not,” says Beatriz del Blanco, first author of the article.
To understand the role of Kmd1a in compartmentalization, the researchers conducted an experiment, in collaboration with Yijun Ruan, former director of the Jackson Laboratory for Genomic Medicine, in which they used a technique that shows how DNA folds inside the cell nucleus, and how it is organized in three dimensions. These data were contrasted with images obtained using super-resolution microscopy. Both approaches indicated that some repressed genes began to be expressed because the barriers that delimit active and inactive areas of the chromatin are weakened after Kdm1a loss.
The laboratory directed by Barco at the IN investigates rare neurological disorders linked to mutations in genes encoding epigenetic regulators. Mutations in the Kmd1a protein during development have been associated with an extremely rare intellectual disability disorder known as Cleft Palate, Psychomotor Delay, Distinctive Facial Features, and Intellectual Disability (CPRF Syndrome). Angel Barco emphasizes the importance of studying rare diseases to expand the limits of knowledge in this field.
This work has been possible thanks to funding from the Spanish Research Agency-Ministry of Science, Innovation, and Universities and the Fundació la Marató de TV3, among other sources.
Source: Institute for Neurosciences CSIC-UMH (in.comunicacion@umh.es)

 Español
Español